Researchers from the Department of Microbiology & Immunology, Dalhousie University, University of Calgary, and Department of Biochemistry and Molecular Biology, University of British Columbia, Canada, worked with human coronaviruses in cell cultures to look for drugs that might prevent accumulation and replication of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Their study titled, “Thiopurines activate an antiviral unfolded protein response that blocks viral glycoprotein accumulation in cell culture infection model,” was published online as a pre-print at the site bioRxiv*.
What is an Unfolded protein response (UPR)?
The researchers explained that viruses that are enveloped, such as the coronavirus, have genetic material that can code for membrane proteins that can be synthesized and modified in the endoplasmic reticulum (ER) before they can be transported to the areas of assembly of the parts of the virion.
If the ER protein folding capacity is overwhelmed by too many virion particles, there is an overload of unfolded proteins in the ER. This triggers an unfolded protein response (UPR). This activates the transcription factor-6 (ATF6), inositol requiring enzyme-1 (IRE1) and PKR-like endoplasmic reticulum kinase (PERK). These can sense that the ER is under stress, and there is thus a synthesis of basic leucine zipper (bZIP) transcription factors.
As the UPR gets activated, the protein folding capacity of the ER is increased. This also triggers the ER-associated degradation (ERAD). All proteins that are not folded properly are brought out of the ER and degraded via the 26S proteasome.
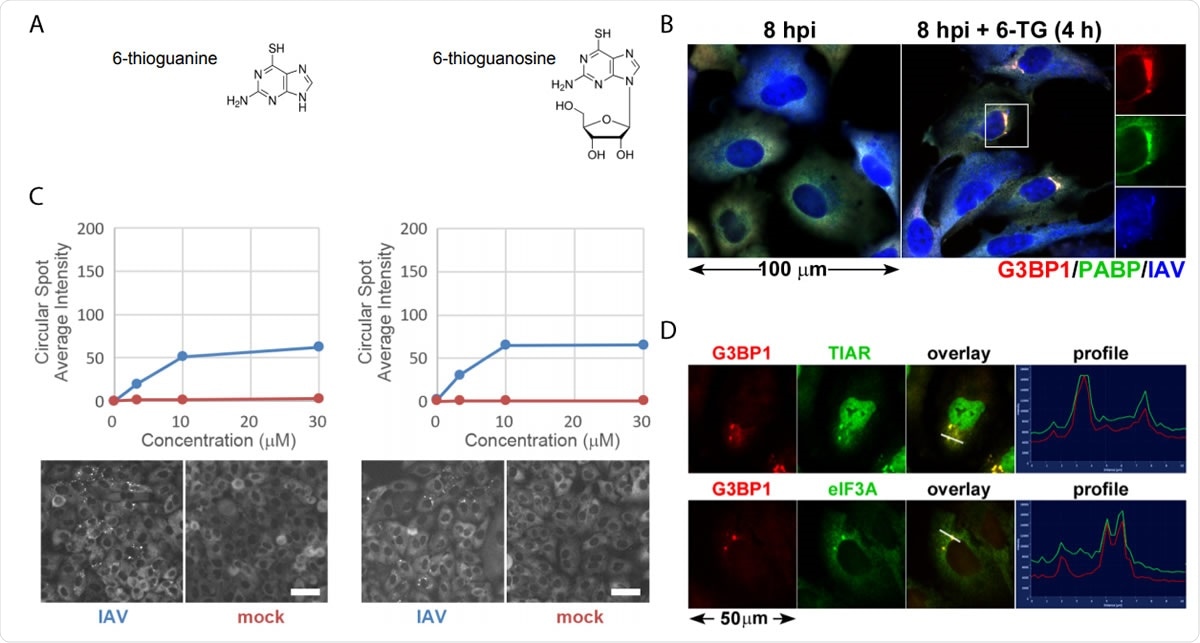
Thiopurine analogs 6-TG and 6-TGo selectively induce stress granules in IAV infected cells. (A) Structural diagrams of small molecules identified in the screen. (B) Quantification of EGFP-G3BP foci formation in IAV-Udorn infected (Blue) or mock (Red) infected cells treated with increasing doses of 6-TG and 6-TGo (top) and representative Cellomics images of EGFP channel of cells treated with 30 µM 6-TG and 6-TGo (bottom). At 4 hpi, cells were treated with 0, 1, 10 and 30 uM doses of thiopurine analogs 6-thioguanine (6- TG) or 6-thioguanosine (6-TGo). At 8 hpi, cells were fixed and stained with Hoeschst 33342. Automated image capture was performed using a Cellomics Arrayscan VTI HCS reader. 15 images were captured for each well and average punctate EGFP-G3BP1 intensity was calculated. (C) A549 cells were infected with IAV-CA/07 at a MOI of 1. At 4 hpi, cells were treated with 6-TG or mock-treated. At 8 hpi, cells were fixed and immunostained with antibodies directed to stress granule marker proteins G3BP1 (red), PABP (green) and a polyclonal IAV antibody (blue) that detects antigens from NP, M1, and HA, followed by staining with Alexa-conjugated secondary antibodies. (D) A549 cells were infected with IAV-CA/07 at a MOI of 1. At 4 hpi, cells were treated with 6-TG (10µM). At 8 hpi, cells were fixed and immunostained with antibodies directed to stress granule marker proteins G3BP1 (red), TIAR (green) and eIF3A (green), followed by staining with Alexa-conjugated secondary antibodies. Images captured on a Zeiss Axioimager Z2 fluorescent microscope. Representative images shown. Scale bars represents 20 µm.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Overwhelming of the ER during viral replication
When the virus particle invades a cell, it tries to replicate fast, and this burdens the ER. The virus releases bursts of glycoproteins that overwhelm the ER. The virus, however, is capable of bypassing the UPR and promotes efficient replication.
Techniques adopted by the Influenza A viruses (IAVs)
The IAV can encode three integral membrane proteins: hemagglutinin (HA), neuraminidase (NA), and matrix protein 2 (M2). While the IAV replication causes selective activation of the UPR, specific mechanisms can activate the UPR but then bypass it to promote effective viral replication. The team explains that the effects of NA and M2 proteins on UPR are not clear, but HA can promote UPR.
Coronaviruses and UPR
Several coronaviruses (CoVs) can activate UPR. This includes the “infectious bronchitis virus (IBV), mouse hepatitis virus (MHV), transmissible gastroenteritis virus (TGEV), human coronavirus (HCoV)-OC43, and SARS-CoV-1.” The whole of the genetic sequence, however, does not react similarly to the CoV replication.
Thiopurine and the virus replication
The team identified two FDA-approved thiopurine analogs called “6- thioguanine (6-TG) and 6-thioguanosine (6-TGo)”. These were found to block IAV and HCoV-OC43 replication when their dose was increased in a graded manner.
Pateamine A and Silvestrol had been tested previously. These two thiopurines, however, were found to disrupt the process of accumulation of viral glycoproteins that could activate the UPR. In the cells that had been treated with 6-TG, the viral glycoprotein synthesis could be partially restored by the chemical inhibition of the UPR.
CoV Spike (S) proteins that are expressed on the surface of the virus showed UPR activation. The S protein from the novel coronavirus or SARS-CoV-2 S also caused UPR activation. 6-TG inhibited the accumulation of full-length S0 or furin-cleaved S2 fusion proteins, they noted. It did not affect the S1 ectodomain. 6-TG could induce UPR that accelerates ERAD-mediated turnover of membrane-anchored S0 and S2 glycoproteins, the team found.
6-mercaptopurine (6-MP) has no such effect
The researchers experimented and found that a chemically similar compound thiopurine 6-mercaptopurine (6-MP) had little effect on UPR and did not affect the replication of IAV HCoV-OC43.
Pondering on the mechanism of UPR induction by the thiopurine compounds 6-TG and 6-TGo, the team wrote that these effects are not likely to be mediated through DNA or RNA incorporation of 6-TG because of several reasons. The first reason is that the stress associated with viral replication does not specifically induce UPR. The second reason is that among viral proteins, the accumulation of glycoproteins and their processing was disrupted selectively. The third reason was that the messenger RNA levels of HA and NA in the IAV were not significantly affected. 6-MP, on the other hand, can be converted into 6-thioguanosine triphosphate but did not induce UPR and had no effects on IAV glycoproteins or OC43 replication.
Conclusions and implications
The team wrote that their data reveals that “UPR-inducing molecules could be effective host-targeted antivirals against viruses that depend on ER processes to support efficient replication.” Induction of UPR by 6-TG and 6-TGo thus could be a novel method by which an antiviral mechanism could be triggered by the host cell itself. This has been a previously unrecognized unique mechanism of action, the team wrote.
They wrote in conclusion, “...these data indicate that 6-TG and 6-TGo are effective host-targeted antivirals that trigger the UPR and disrupt accumulation of viral glycoproteins.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Thiopurines activate an antiviral unfolded protein response that blocks viral glycoprotein accumulation in cell culture infection model Patrick Slaine, Mariel Kleer, Brett Duguay, Eric S. Pringle, Eileigh Kadijk, Shan Ying, Aruna D. Balgi, Michel Roberge, Craig McCormick, Denys A. Khaperskyy bioRxiv 2020.09.30.319863; doi: https://doi.org/10.1101/2020.09.30.319863
- Peer reviewed and published scientific report.
Slaine, Patrick D., Mariel Kleer, Brett A. Duguay, Eric S. Pringle, Eileigh Kadijk, Shan Ying, Aruna Balgi, Michel Roberge, Craig McCormick, and Denys A. Khaperskyy. 2021. “Thiopurines Activate an Antiviral Unfolded Protein Response That Blocks Influenza a Virus Glycoprotein Accumulation.” Edited by Stacey Schultz-Cherry. Journal of Virology 95 (11). https://doi.org/10.1128/jvi.00453-21. https://journals.asm.org/doi/10.1128/JVI.00453-21.