An international team of researchers has presented a convenient, low-cost serology test to detect antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that could potentially be made available universally.
SARS-CoV-2 is the agent responsible for the current coronavirus disease 2019 (COVID-19) pandemic that continues to threaten global public health and the economy.
Alain Townsend (University of Oxford) and colleagues report that the Hemagglutination Test (HAT) detects antibodies against the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein with a sensitivity of 90% and specificity of 99%.
The RBD is a subdomain found at the tip of the spike protein – the main structure the virus uses to bind to and enter human host cells.
The HAT can detect rising titers of RBD-specific antibodies within the first five days of infection, correlates well with an established commercial test, and can be applied in point-of-care (POC) testing.
This versatile, cost-effective test does not require specialist equipment or centralized laboratory facilities and could be made available to low- and middle-income countries.
Furthermore, the test can be lyophilized for ease of shipping, say Townsend and colleagues, who have now scaled-up production of this reagent to one gram – enough to provide ten million tests, at a cost of around 0.27 UK pence per test well.
“Aliquots of this reagent are ready to be supplied to qualified groups anywhere in the world that need to detect antibodies to SARS-CoV-2, but do not have the facilities for high-throughput commercial tests,” writes the team.
A pre-print version of the paper is available in the server medRxiv*, while the article undergoes peer review.
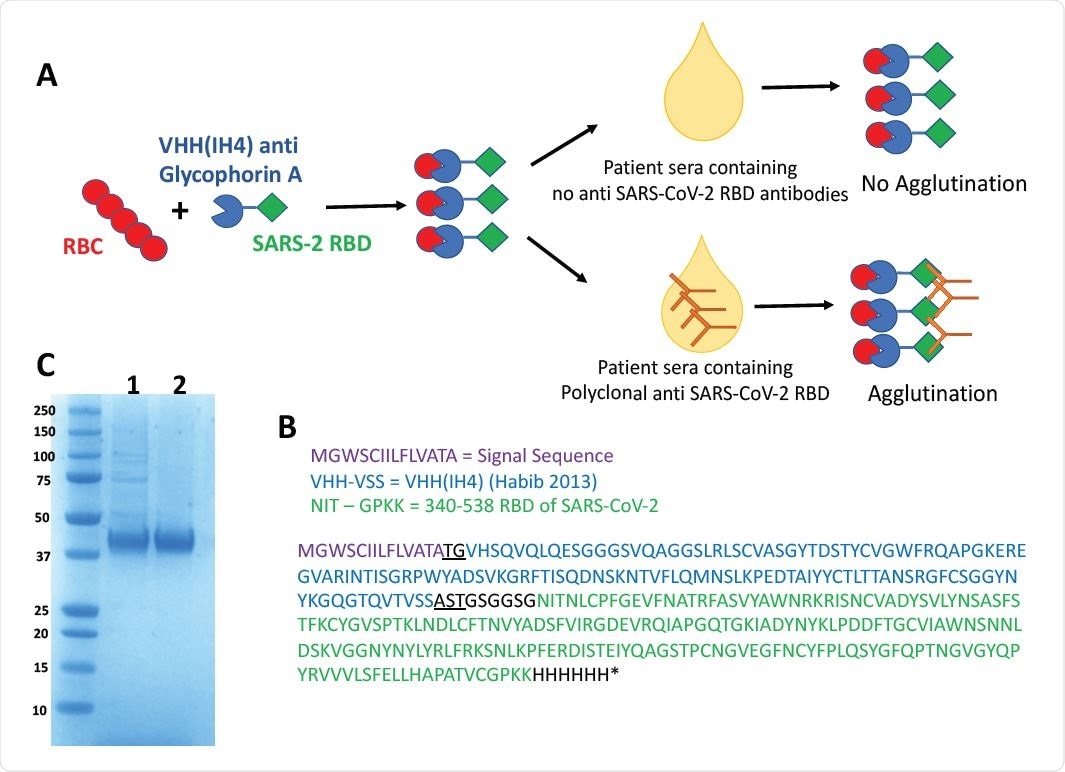
Haemagglutination Test (HAT) for detection of antibodies to SARS-CoV-2 Receptor Binding Domain. A) Concept of the HAT B) Sequence of VHH(IH4)-RBD fusion protein. Residues underlined are encoded by cloning sites AgeI (TG) and SalI (AST). The codon optimised cDNA sequence is shown in supplementary Information C) SDS-PAGE gel of purified VHH(IH4)-RBD proteins. Three micrograms of protein were run on 4-12% Bolt Bis-Tris under reducing conditions. 1: IH4-RBD produced in house in Expi293F cells, 2: IH4-RBD produced by Absolute Antibody, Oxford in HEK293 cells.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Affordable serology tests are urgently needed
Since the first cases of SARS-CoV-2 were identified in Wuhan, China, late last year, the virus has spread at an unprecedented rate and had a devastating effect on global public health and the worldwide economy.
“The appearance of such a new highly contagious virus will probably not be a unique occurrence in the decades ahead,” said Townsend and colleagues. “One of the lessons learned is the importance of developing affordable serological tests for the detection of immune responses to SARS-CoV-2.”
Such tests are crucial for determining seroconversion rates in populations, detecting seroconversion following vaccination, and for assessing whether antibody levels may be protective against COVID-19.
Several high-performance commercial antibody tests have been described, but they are not widely available to low- and middle-income countries, since they require costly centralized laboratory facilities.
Red cell agglutination tests, which involving linkage of a reporter molecule to the surface of red cells, have a long and distinguished history in blood typing and a wide variety of serology applications. They are inexpensive, and their application does not require specialist technology.
Furthermore, “in the recent era, the linkage of an antigen to the red cell surface has become easier with the possibility of fusing a protein antigen sequence with that of a single domain antibody or nanobody specific for a molecule on the red cell surface,” says the team.
What did the researchers do?
The researchers have applied this concept to provide a convenient HAT test for the detection of SARS-CoV-2 spike RBD-specific antibodies.
In order to link the spike RBD to red blood cells, the researchers used the nanobody IH4, which is specific for a conserved epitope on the transmembrane protein glycophorin A.
The team reports that in a formal assessment of sensitivity and specificity, HAT demonstrated a sensitivity of 90% and specificity of 99% for the detection of antibodies following a PCR-confirmed diagnosis of SARS-CoV-2 infection.
HAT correlated well with the commercially available Siemens Atellica Chemiluminescence test that detects spike RBD-specific antibodies.
Interestingly, says the team, the HAT titrations demonstrated slightly superior performance over the Siemens test when it was applied to stored plasma samples taken from donors within the first five days of their hospital admission.
In this scenario, HAT detected antibodies in 86% of samples from PCR-diagnosed donors, while the Siemens test detected 74%. HAT also demonstrated 100% specificity when applied to control plasma samples taken from healthy patients or those with sepsis.
“In situations of high clinical suspicion, the HAT could potentially have a place as a helpful test to support the diagnosis of COVID-19 by detecting a rising titer of antibodies to the RBD during hospital admission,” write the researchers.
The HAT might also be useful for detecting seroconversion following vaccination and for the identification of potential convalescent donors with high titer plasma that could potentially be used as a treatment.
Enough reagent for 10 million tests is available to the world
The team also says lyophilized IH4-RBF reagent that was sent to New Delhi performed well in POC testing of finger-prick samples. However, further evidence is needed to confirm that the sensitivity and specificity are comparable to those seen with tests of stored plasma samples.
“This will need to be done in field conditions, which is planned,” write Townsend and colleagues.
Finally, the team says: “We have produced one gram of the developing IH4-507 RBD reagent (enough for ten million test wells) and offer to ship lyophilized aliquots of this material (sufficient for 10,000 tests) anywhere in the world, free of charge, for use as a research reagent for serological studies of COVID-19.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Townsend A, et al. A haemagglutination test for rapid detection of antibodies to SARS-CoV-2. medRxiv, 2020. doi: https://doi.org/10.1101/2020.10.02.20205831
- Peer reviewed and published scientific report.
Townsend, Alain, Pramila Rijal, Julie Xiao, Tiong Kit Tan, Kuan-Ying A. Huang, Lisa Schimanski, Jiandong Huo, et al. 2021. “A Haemagglutination Test for Rapid Detection of Antibodies to SARS-CoV-2.” Nature Communications 12 (1). https://doi.org/10.1038/s41467-021-22045-y. https://www.nature.com/articles/s41467-021-22045-y.