A new study has shown that severe coronavirus disease 2019 (COVID-19) is associated with unique B cell receptor (BCR) signatures and multiclonal neutralizing responses against the causative agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The research team, from Israel, Australia, and the United States, say that these BCR signatures and neutralizing responses are relatively common in the population.
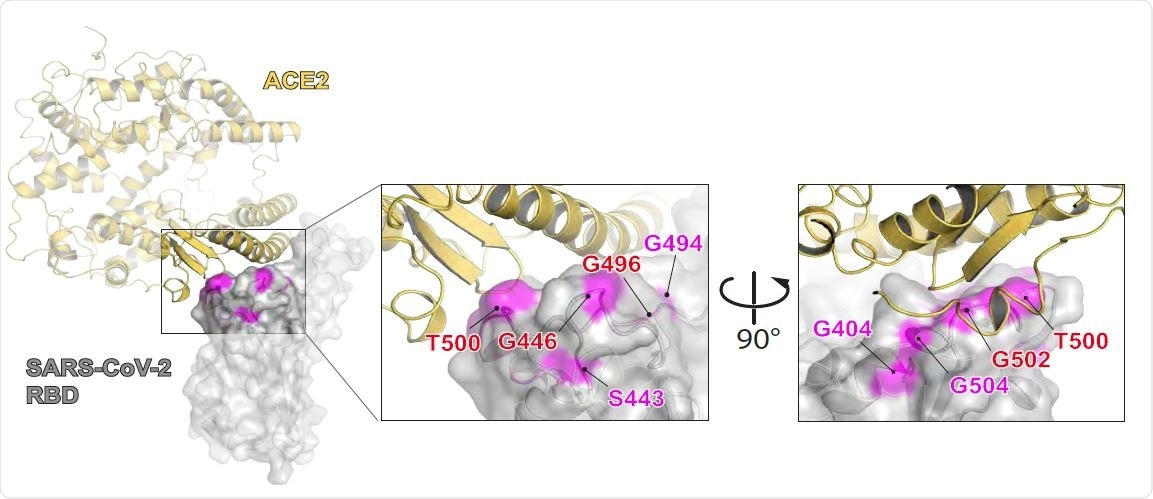
Six neutralizing antibodies isolated from convalescent donors who had recovered from severe disease exhibited neutralizing activity that targeted three different sites on the Spike protein of SARS-CoV-2. The spike protein is the main structure the virus uses to bind to the human receptor angiotensin-converting enzyme 2 (ACE2) and gain entry to host cells.
The researchers also found that combinations of neutralizing antibodies that target different immune-sites effectively blocked the spread of live SARS-CoV-2.
This is the first study to demonstrate inhibition of the virus using combinations of SARS-CoV-2 neutralizing antibodies, says the team.
“Our data support the use of combination antibody therapy to prevent and treat COVID-19,” writes Natalia Freund (Tel Aviv University) and colleagues.
A pre-print version of the paper is available in the server bioRxiv*, while the article undergoes peer review.
Activity of anti-SARS-CoV-2 mAbs in ELISA. CoV01 and CoV02 mAbs, respectively, binding to SARS-CoV-2 RBD (left) and Spike trimer (right). The color-code is indicated to the right of each graph.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
How neutralizing antibodies influence disease progression is not yet clear
During the course of COVID-19, patients typically produce antibodies against the virus, antibodies that have proven to be protective in studies of animal models and human cell lines.
Although scientists generally agree that neutralizing antibodies (nAbs) are generated in response to SARS-CoV-2 infection, it remains unclear exactly how they influence disease progression.
“The link between COVID-19 clinical manifestations and SARS-CoV-2 antibody responses is still not clear,” said Freund and team.
Furthermore, it is unknown whether these antibody responses affect future exposure to the virus and protect against re-infection.
Exploring antibody responses to mild versus severe disease
To explore the differences in antibody responses to mild versus severe cases of COVID-19, the researchers assessed B cell responses among 18 convalescent donors 6 weeks following SARS-CoV-2 infection.
Eight of the patients had required hospitalization for severe disease, while 10 had only experienced mild symptoms or were asymptomatic (termed “mild disease” hereafter).
The researchers report that severe and not mild disease is correlated with robust, inhibitory antibody responses against the receptor-binding domain (RBD) of the SARS-CoV-2 Spike protein.
All patients who had developed severe symptoms of infection had generated similarly high titers of SARS-CoV-2-specific immunoglobulin G (IgG), whereas patients who had mild disease generated titers ranging from high to non-detectable.
“Although this indicates that high titers of SARS-CoV-2 antibodies are not responsible for lack of COVID-19 symptoms, these antibodies and memory B cells developing during severe disease may prevent future re-infection,” writes the team.
BCR sequencing revealed that two immunoglobulin heavy chain variable region (VH) genes, namely VH3-38 and VH3-53, were enriched in cases of severe infection. The VH3-53 neutralizing antibody has previously been described as being dominant in immune responses to SARS-CoV-2.
However, the nAbs isolated from two donors who had experienced severe disease did not originate from these VH genes. The neutralizing response exhibited by these two donors was multiclonal, with several neutralizing clones targeting three different sites on the Spike protein.
Antibodies targeted the ACE 2 binding site as well as sites lying outside of ACE2
Both donors produced antibodies against the ACE2 binding site of the Spike RBD, and contact residues of the nAb TAU-2230 were predicted to partially overlap with the ACE2 binding site.
The researchers say this points to the ACE2 binding site as a significant neutralizing determinant that is targeted by antibodies during the infection.
The nAbs TAU-1109 and TAU-2212 also targeted an additional two neutralizing determinants lying outside of the ACE2 binding site.
The epitopes for these nAbs, which are yet to be identified, may represent new sites of SARS-CoV-2 vulnerability that could be potential targets for vaccines, say the researchers.
Finally, the team showed that using combinations of nAbs targeting different immune sites effectively blocked SARS-CoV-2 infection.
Analysis of uninfected, healthy BCR repertoires showed that the precursors that give rise to these nAbs are abundant in the naïve population, making up 2.7% of all VH and joining (JH) genes.
However, Freund and colleagues say that somewhat counterintuitively, many donors who only experienced mild disease did not develop IgGs that could stop the Spike RBD binding to ACE2.
“This suggests that while most individuals have SARS-CoV-2 nAb precursors in their BCR repertoires, a single dose vaccine may not be sufficient to elicit protective anti-SARS-CoV-2 immunity,” they warn.
What are the implications of the study?
The researchers say the study shows that severe COVID-19 is associated with unique BCR signatures and multi-clonal neutralizing responses that are relatively frequent in the population.
The team also says the data support the use of combination antibody therapy for the prevention and treatment of COVID-19.
“This is the first demonstration that combinations of neutralizing anti-SARS-CoV-2 nAbs can effectively block the spread of live SARS-CoV-2,” write the researchers.
“The combinations examined in our study can be further tested in clinical settings as a useful means of prevention and therapy of COVID-19,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Freund N, et al. Multi-Clonal Live SARS-CoV-2 In Vitro Neutralization by Antibodies Isolated from Severe COVID-19 Convalescent Donors. bioRxiv, 2020. doi: https://doi.org/10.1101/2020.10.06.323634
- Peer reviewed and published scientific report.
Mor, Michael, Michal Werbner, Joel Alter, Modi Safra, Elad Chomsky, Jamie C. Lee, Smadar Hada-Neeman, et al. 2021. “Multi-Clonal SARS-CoV-2 Neutralization by Antibodies Isolated from Severe COVID-19 Convalescent Donors.” Edited by Kanta Subbarao. PLOS Pathogens 17 (2): e1009165. https://doi.org/10.1371/journal.ppat.1009165. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009165.