Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which emerged in Wuhan, China, in late 2019 and spread worldwide, causing a pandemic of unprecedented proportions. So far, the pandemic has affected over 200 countries, with 36.44 million confirmed cases and over 1.06 million fatalities globally.
Given the global health crisis the pandemic has caused, scientists worldwide are struggling to find a vaccine or drug to fight against the SASR-CoV-2 virus. According to clinical studies, the ORF8 protein of the SARS-CoV-2 virus has been shown to be associated with severe COVID-19 symptoms. However, not much is known about the exact function of the ORF8 protein.
What do we know about the SARS-CoV-2 ORF8 protein?
Studies have shown that the SARS-CoV-2 ORF8 protein shows high homology with ORF8 of bat coronavirus. Mutant SARS-CoV-2 viruses that lack ORF8 protein were reported to be less likely to cause severe disease. The ORF8 protein is said to be highly immunogenic, and anti-ORF8 antibodies are produced in the early stage of COVID-19 infection. Also, significant T-cell response to ORF8 has been observed in recovered COVID-19 patients. Thus, ORF8 is said to be closely linked to the symptoms caused by the SARS-CoV-2 virus.
X-ray crystallization studies have shown the 3D structure of ORF8 and that the protein affects the human immune system. In order to do a functional analysis of ORF8, it is essential to mass-produce the ORF8 protein with the correct 3D structure.
Is large-scale production of the ORF8 protein possible?
Researchers from the Japan Advanced Institute of Science and Technology (JAIST) and Ishikawa Prefectural University, Japan, recently attempted to synthesize the ORF8 protein with the help of a chemical-inducible protein production system using tobacco BY-2 cells. This study has been published demonstrated in the preprint server, bioRxiv.*
The team of researchers generated an ORF8-producing line using the Agrobacterium method and produced 8.8 ± 1.4 mg of ORF8 per L of culture medium. Using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and nuclear magnetic resonance (NMR) analysis, they confirmed that the ORF8 protein they produced in tobacco BY-2 cells is in its dimeric form with a 3D structure, unlike that produced in Escherichia coli (E. coli). Additionally, the ORF8 produced using this system was N-glycosylated.
ORF8 produced with its 3D structure intact can help in functional analysis and vaccine development
In this study, the team produced structurally intact ORF8 protein in a tobamovirus (ToMV)-mediated production system. The authors say that the purification of ORF8 could be simplified by releasing the protein into the culture medium that has very little plant-derived contaminants. According to the authors, the advantage of using plants for protein production is that the risk of contamination with endotoxins or pathogens is nil compared to protein production using other organisms such as E. coli.
"The advantage of protein production using plants is that there is no risk of contamination with endotoxins and pathogens compared to production using other organisms."
The findings of the study prove that it is possible to mass-produce structural ORF8 protein with high efficiency. Since the ORF8 produced had the 3D structure intact, it can be used in functional analysis and the production of antibodies and vaccines that target the SARS-CoV-2 ORF8 protein in infected individuals.
According to the authors, the system they have developed using tobacco BY-2 cells is a very efficient chemical-inducible protein production system. They have utilized the very high protein production capacity of ToMV. The researchers claim that their work helped produce ORF8 with the correct 3D structure using an efficient production system in tobacco BY2 cells. They hope that the ORF8 produced by them will advance functional analysis of ORF8 and will help the development of antibodies and vaccines against the ORF8 protein.
"Mature ORF8 has seven cysteines, six of which form three intramolecular disulfide-bond pairs and one forms an intermolecular disulfide-bond for dimerization. In addition, one N-glycosylation site is predicted for ORF8. Our production system is expected to be suitable for uniform ORF8 protein production with these structural features."
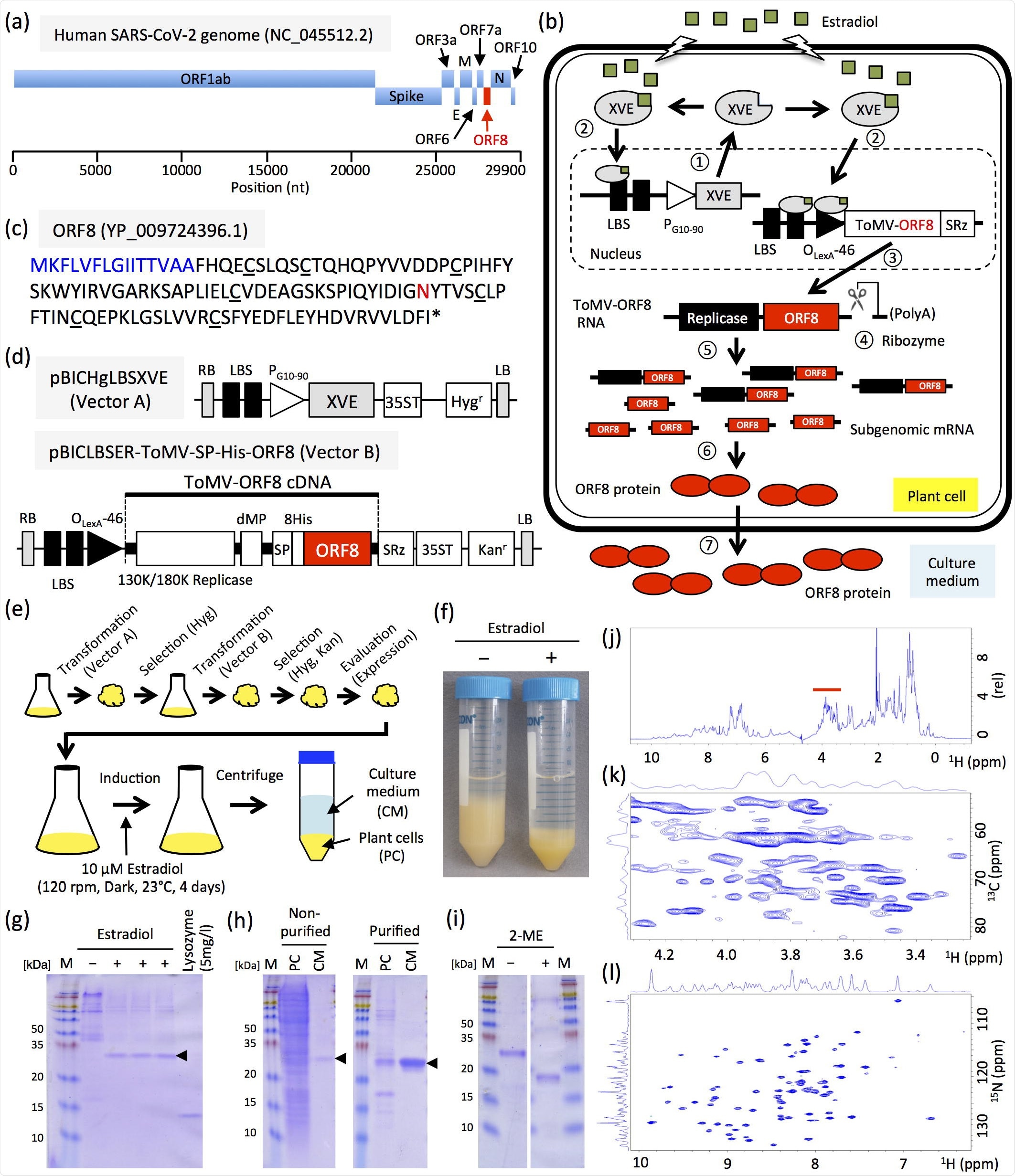
Production and characterization of ORF8. (a) Schematic representations of SARS-CoV-2 genome. E, M, and N indicate envelope protein, membrane glycoprotein, and nucleocapsid phosphoprotein, respectively. (b) Schematic overview of the chemical-inducible ToMV-mediated expression-system. Step 1: Constitutive expression of XVE, a transcriptional activator. Step 2: XVE binds to estradiol and gets activated. Step 3: Activated XVE binds to the OLexA-46 promoter or LBS to promote transgene transcription. Step 4: Non-viral sequences that interfere with the replication of the viral vector are removed by ribozymes. Step 5: Viral vector replication. Step 6: Translation of ORF8 from subgenomic RNA. Step 7: Translocation of ORF8. (c) Amino acid sequence of ORF8. Blue and red letters indicate signal peptide and N-glycosylation site, respectively. Underbars indicate cysteine. (d) Schematic representations of ToMV-mediated chemical-induced expression plasmids. XVE, transcriptional activator that responds to estrogen; SP, signal peptide of Arabidopsis chitinase; 8 His, 8× His-tag; LBS, LexA binding site; PG10-90, synthetic constitutive promoter; PLexA-46, fusion promoter controlled by XVE; dMP, partial movement protein; SRz, ribozyme sequence from tobacco ringspot virus satellite RNA; 35ST, 35S terminator; RB, right border; and LB, left border. Hygr and Kanr indicate expression cassette of hygromycin and kanamycin resistance genes, respectively. (e) Generation of ORF8-producing cell line and induction of ORF8 production. (f) Four days after 17β-estradiol induction of suspension-cultured tobacco BY-2 cells; + and − indicate with or without 17β-estradiol, respectively. (g) Detection of ORF8 from 7.5 µL cultured medium by Coomassie Brilliant Blue staining. + and − indicate with or without 17β-estradiol, respectively. Arrowhead indicates ORF8. Lysozyme (5 mg/L) indicates loading control. (h) Non-purified and purified ORF8. PC and CM indicate crude extracts of plant cells and culture medium, respectively. (i) Molecular property of ORF8. + and − indicate with or without 2-mercaptoethanol (2ME), respectively. M, protein size marker. Numbers indicate molecular weights (kDa). (j) 1 H NMR spectra of unlabeled ORF8. Red line indicates the glycan signal appearing region. (k) 1 H-13C HSQC of unlabeled ORF8 protein. An expanded view of the glycan signal appearing region is shown. (l) 1 H-15N HSQC of 15N-labeled ORF8.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Production of ORF8 protein from SARS-CoV-2 using an inducible virus-mediated expression-system in suspension-cultured tobacco BY-2 cells Tomohiro Imamura, Noriyoshi Isozumi, Yasuki Higashimura, Shinya Ohki, Masashi Mori bioRxiv 2020.10.07.325910; doi: https://doi.org/10.1101/2020.10.07.325910, https://www.biorxiv.org/content/10.1101/2020.10.07.325910v1
- Peer reviewed and published scientific report.
Imamura, Tomohiro, Noriyoshi Isozumi, Yasuki Higashimura, Shinya Ohki, and Masashi Mori. 2021. “Production of ORF8 Protein from SARS-CoV-2 Using an Inducible Virus-Mediated Expression System in Suspension-Cultured Tobacco BY-2 Cells.” Plant Cell Reports 40 (3): 433–36. https://doi.org/10.1007/s00299-020-02654-5. https://link.springer.com/article/10.1007/s00299-020-02654-5