A large-scale randomized trial has recently been conducted by the World Health Organization (WHO) to evaluate the efficacy of four repurposed antiviral medicines, including Remdesivir, Hydroxychloroquine, Lopinavir, and Interferon, currently used to treat hospitalized coronavirus disease 2019 (COVID-19) patients.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Unfortunately, the trial results reveal that none of these medicines effectively reduce mortality, initiation of ventilation, and duration of hospital stay. The study is currently available on the medRxiv* preprint server.
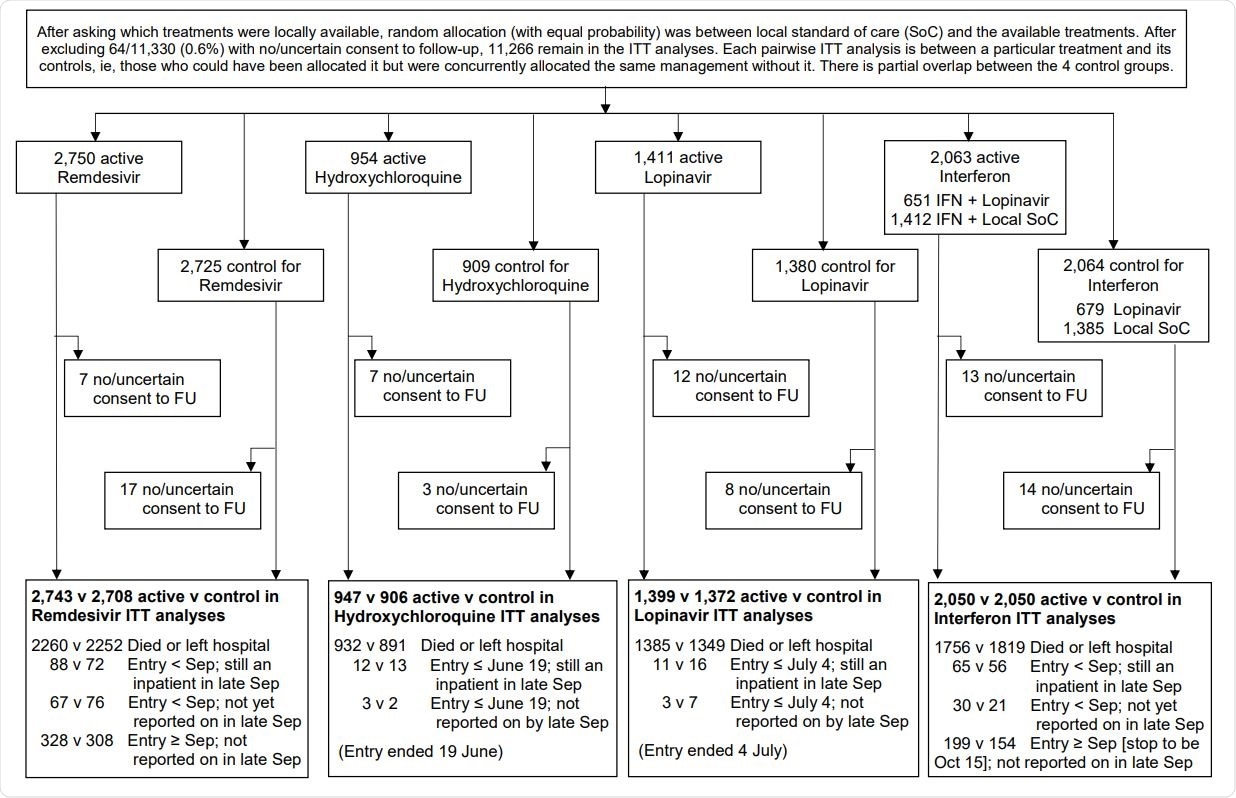
WHO Solidarity Trial – information to October 4, 2020 on entry, follow-up (FU) and intent-to-treat (ITT) analyses
Background information
Since the emergence of the COVID-19 pandemic, many antiviral drugs have been repurposed to treat severely affected COVID-19 patients. Of these drugs, Remdesivir, Hydroxychloroquine, Lopinavir, and Interferon have shown promising outcomes in many studies. To evaluate the efficacy of these four drugs in reducing COVID-19 related morbidity and mortality, a large, multinational, randomized trial involving hospitalized COVID-19 patients has been conducted by a WHO expert team in March 2020.
The trial protocols
The trial involved a total of 11,266 COVID-19 patients who were admitted to 405 hospitals in 30 countries. The patients were randomly divided into different drug groups. Precisely, 2,750 patients received Remdesivir, 954 received Hydroxychloroquine, 1,411 received Lopinavir, 651 received Interferon, and Lopinavir, 1412 received only Interferon, and 4088 did not receive any study drug (control group). No placebos were used in this trial.
The primary objective of the trial was to assess in-hospital mortality. In addition, initiation of ventilation and duration of hospital stay were assessed as secondary objectives.
Findings of the trial
The trial findings revealed that none of the study drugs had beneficial effects on hospitalized COVID-19 patients in terms of reducing mortality, initiation of ventilation, and hospitalization duration.
Remdesivir initially developed for treating Ebola and hepatitis C patients, has been repurposed for COVID-19 treatment because of its ability to inhibit viral replication. A trial conducted by the National Institutes of Health (NIH) has previously shown that Remdesivir can moderately reduce the recovery time in COVID-19 patients. Based on this finding, the US Food and Drug Administration (FDA) has granted emergency authorization for Remdesivir use on May 1, 2020. Presently in the United States, Remdesivir is used routinely for treating hospitalized COVID-19 patients irrespective of disease severity.
Although no effect on mortality has been observed in the NIH’s trial, a study published in the New England Journal of Medicine has shown that Remdesivir reduced mortality by 70% in COVID-19 patients receiving low-flow oxygen. The study also showed that Remdesivir effectively reduces recovery time and disease progression.
Previous studies have revealed controversial findings regarding Hydroxychloroquine and Lopinavir, with most showing no specific or significant efficacy with these drugs when treating hospitalized COVID-19 patients. Regarding Interferon, no large-scale trials assessing the mortality reduction effects have been reported.
According to the WHO experts, the “unpromising” findings of the current trial “suffice to refute early hopes” created by small-scale or non-randomized trials that any of the study drugs “will substantially reduce inpatient mortality, initiation of ventilation, or hospitalization duration.”
They mentioned in the manuscript that mortality related findings the trial showed are in line with the findings of all major clinical trials.
To study the efficacy of other therapeutic drugs, such as immunomodulators and monoclonal antibodies explicitly targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the trial is currently recruiting about 2,000 patients per month.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources