Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus behind the ongoing global pandemic, causes coronavirus disease 2019 (COVID-19). This disease can cause acute respiratory distress syndrome (ARDS), pulmonary inflammation, respiratory failure, and even death. Despite the high level of morbidity and mortality associated with COVID-19, most people infected with SARS-CoV-2 survive the illness. However, whether they still have immunity against SARS-CoV-2 following their recovery is still not clear. The durability of immunity plays a crucial role in minimizing the reinfection risk in millions of people recovering from SARS-CoV-2 infection.
Several studies have shown that SARS-CoV-2 specific antibodies deplete over time following infection and recovery. This raises concerns that humoral immunity against SARS-CoV-2 is not durable. If immunity does wane over time, millions of people who have recovered from the infection might be at risk for reinfection.
Although some studies have reported that memory B cells can provide durable humoral immunity even when serum antibody titers decline, not much is known about the frequency and phenotype of SARS-CoV-2-specific memory B cells developed in response to severe or mild infection. B cells specific to the SARS-CoV-2 Spike (S) protein have been isolated from patients having very low antibody titers. However, the relatively low frequency of these cells hinders further characterization.
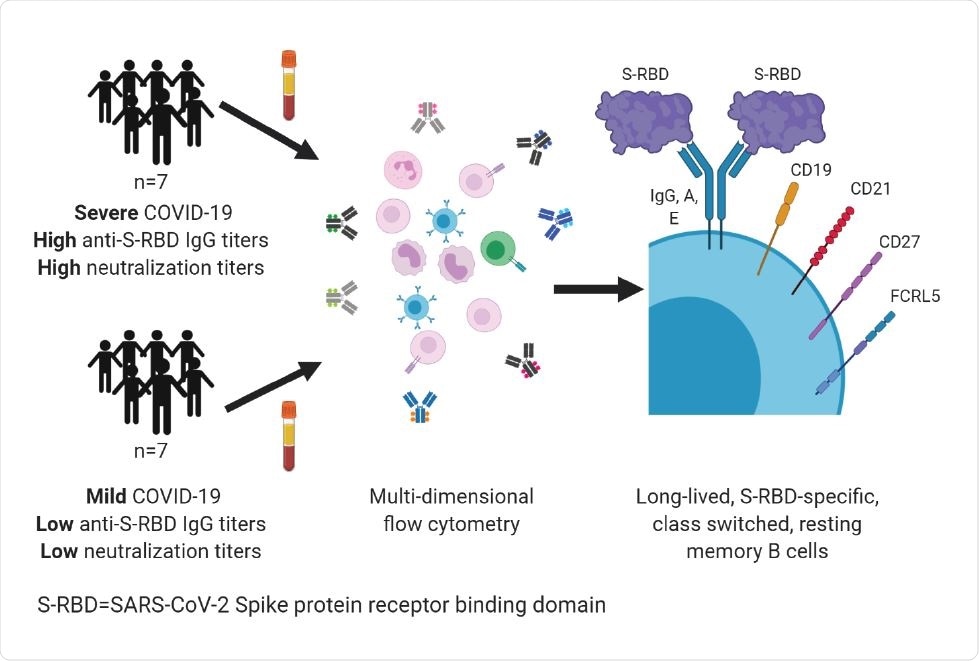
Flow cytometric analysis helps quantify B cells specific to SARS-CoV-2 protein receptor-binding domain

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Researchers from the Johns Hopkins University and The Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA, investigated the durability of B cell immunity following SARS-CoV-2 infection and recovery in their recent work published on the preprint server medRxiv*.
The researchers developed a precise and sensitive flow cytometry-based assay for quantitating B cells specific to the SARS-CoV-2 protein receptor-binding domain (S-RBD). They also designed a cell surface phenotyping panel to characterize these B cells. They focused on B cells specific to S-RBD because most human monoclonal antibodies that neutralize viruses target this domain. The neutralizing activity has been linked to protection against reinfection by similar coronaviruses in animal models of SARS-CoV-2 infection.
They performed a multi-dimensional flow cytometric analysis of memory B cells specific to the S-RBD. Their study cohorts comprised ambulatory COVID-19 patients who had a mild disease as well as hospitalized COVID-19 patients with moderate or severe disease. The analyses were performed at a median of 54 (39-104) days following the onset of symptoms.
Resting memory B cells are the most abundant S-RBD-specific memory B cells detected in the study
The researchers detected S-RBD-specific memory B cells in 13 out of 14 patients, including 4 of the 5 participants with deficient levels of anti-S-RBD IgG and neutralizing antibodies in plasma. Resting memory B cells (rMBC) constituted the largest proportion of S-RBD-specific memory B cells detected in both study cohorts. A marker of functional memory, FCRL5, was drastically upregulated on S-RBD-specific rMBC, especially in patients with mild disease.
“FCRL5, a marker of a functional memory response when expressed on antigen-specific rMBC, was dramatically upregulated on S-RBD-specific rMBC, particularly after mild infection.”
Most patients develop S-RBD-specific B cells that phenotypically resemble B cells induced by vaccination
To summarize, the researchers demonstrated that S-RBD-specific memory B cells are developed in most SARS-CoV-2-infected patients, including those with mild symptoms or very low levels of anti-S-RBD IgG and neutralizing antibodies in plasma. The least abundant S-RBD specific memory B cells in both cohorts were atypical memory B cells (atyMBC).
Based on these data, the team concluded that most individuals infected with SARS-CoV-2 develop S-RBD-specific, class-switched memory B cells that phenotypically resemble germinal center-derived B cells that are induced following vaccination. This provides evidence for durable B cell-mediated immunity against the SARS-CoV-2 virus post recovery from mild to severe COVID-19. The authors believe that their findings offer a standard against which B cell responses to new SARS-CoV-2 vaccines could be compared in the future.
“These data have implications for risk of reinfection after recovery from COVID-19, and also provide a standard against which B cell responses to novel SARS-CoV-2 vaccines could be compared.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Durable SARS-CoV-2 B cell immunity after mild or severe disease Clinton O. Ogega, Nicole E. Skinner, Paul W. Blair, Han-Sol Park, Kirsten Littlefield, Abhinaya Ganesan, Pranay Ladiwala, Annukka AR Antar, Stuart C. Ray, Michael J. Betenbaugh, Andrew Pekosz, Sabra L. Klein, Yukari C. Manabe, Andrea L. Cox, Justin R. Bailey medRxiv 2020.10.28.20220996; doi: https://doi.org/10.1101/2020.10.28.20220996, https://www.medrxiv.org/content/10.1101/2020.10.28.20220996v1
- Peer reviewed and published scientific report.
Ogega, Clinton O., Nicole E. Skinner, Paul W. Blair, Han-Sol Park, Kirsten Littlefield, Abhinaya Ganesan, Santosh Dhakal, et al. 2021. “Durable SARS-CoV-2 B Cell Immunity after Mild or Severe Disease.” The Journal of Clinical Investigation 131 (7). https://doi.org/10.1172/JCI145516. https://www.jci.org/articles/view/145516.