The rapid rise to dominance of the D614G spike mutation-carrying strains of the SARS-CoV-2 virus wherever it has emerged worldwide has spurred much research into the possible survival advantage associated with this genetic variant.
Three Variants
The three major circulating variants of SARS-CoV-2 show 1) D614G mutation in the spike protein, 2) G251V in the non-structural protein (NS3), and 3) L84S in the ORF8 protein. The current study focuses on the D614G substitution.
The spike glycoprotein of SARS-CoV-2 comprises three parts: a large part that protrudes from the virus surface, an anchoring segment and a small intracellular tail. The protruding part itself (also known as the ectodomain) has two parts, S1 and S2, which bind to and fuse with the host cell, respectively.
The Two Subunits
The S1/S2 junction has a cleavage site that allows the virus to shed the S1 subunit, while the S2 has another cleavage site that cleaves the S2 region. The S1, in the functional protein, is the site of the receptor-binding domain (RBD), which can adopt an ‘open’ or ‘closed’ conformation, depending on its orientation.
In the ‘open’ state, the RBD exposes the binding site that engages the host cell receptor, the angiotensin-converting enzyme (ACE2), at a peptidase domain. This attachment step holds the virus to the host cell. This is followed by activation of the S1/S2 cleavage site by the host protease, which causes the S1 subunit to shed.
Again, the S2 subunit undergoes cleavage, triggering a significant change in conformation in S2. This causes the fusion peptide of the virus to insert into the cell membrane of the host cell, forming a spike protein bridge between the two. The presence of a hairpin loop in S2 brings both close together, such that the viral genome is internalized and enters the host cell to produce viral infection.
The polybasic cleavage site at S1/S2 ensures that the host furin protease can preactivate the spike protein of SARS-CoV-2 while it is being packaged, following its synthesis within the infected host cell. This means that the virus no longer requires the host protease to undergo fusion, speeding up the infection of further cells after the release of new virions from the infected target cell.
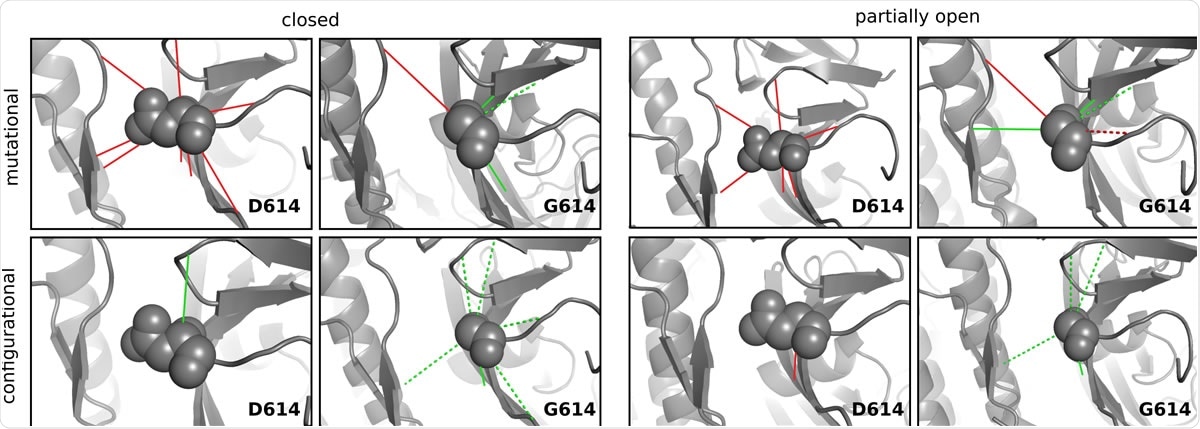
Frustration in the local interaction energies of SD614 and SG614 variants. Shown are the mutational (top panel) and configurational (bottom panel) frustrations exist in the inter-residue interactions formed by aspartate or glycine at 614th position. Minimally and highly frustrated interactions are indicated by green and red lines, respectively. Watermediated interactions are represented as dashed lines and the variant residue is shown as sphere. Results for closed and partially open conformation were shown for a protomer (chain ID: A) in the trimer and similar patterns are observed for the other two protomers (Supplementary Table S1).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Effect of Spike D614 Mutation
Any mutation in the spike protein that alters this first step in viral infection can therefore bring about a change in the transmission dynamics of the virus or its pathogenicity. In the original strain, position 614 bears the amino acid aspartate, which in the D614G is replaced by glycine. This is known to confer higher infectivity on the virus.
At the same time, the presence of glycine rather than aspartate interferes with the formation of a salt bridge between this aspartate and lysine at position 854 of the next protomer. This disruption may thus allow the open state to become more frequent in the mutant.
A cryo-electron microscopy (cryo-EM) study confirms that the presence of glycine at this position stabilizes the spike protein and prevents the S1 subunit from being shed too early.
Frustration Index and the D614 Mutation
In the current study, the researchers looked at a measure called the frustration index (FI). They found that in the ancestral variant D614, the frustration is high in both the ‘closed’ and ‘partly open’ states.
In G614, however, the frustration is neutral in both conformations. This finding shows that the D614G mutation produces a more stable linkage between the S1 and S2 subunits of each protomer, as well as between the subunits of adjacent protomers, thus increasing the stability profile of the trimeric spike protein.
Again, the aspartate in D614 makes contact with other residues within the same protomer and between protomers, using electrostatic or water-mediated linkages. In a total of 8 linkages, all were found to be highly frustrated. With glycine replacing aspartate (G614), in both states, glycine has three minimally frustrated contacts within and between protomers, with one additional contact in the ‘partly closed’ conformation. The fact that glycine creates more minimally frustrated contacts shows improvement in the environment around position 614 compared to aspartate.
Apart from this, they looked at how frustrated the original contact was between two amino acids compared to other possible contacts between the two residues involved – the configurational frustration. This analysis showed that in the ‘closed’ state, the aspartate has only one favorable contact within the same protomer vs the six such contacts created within the same protomer and adjacent protomers, with glycine in this position.
When in the ‘partly open’ conformation, there is only one very frustrated contact with aspartate at 646, but glycine makes four minimally frustrated contacts.
Naturally, reducing frustration in the local energies of interaction will have a marked effect on the thermodynamic stability of the trimeric spike. The total free energy of the trimer shows a significant reduction in both conformations. This thus enhances the stability of the trimeric spike.
Reduced Free Energy Improves Infectivity
The researchers sum up, “Overall, calculations of single residue, mutational and configurational frustrations reveal that glycine substitution modified local interaction energy in the favorable direction.”
The result is that the functional spike becomes more available, which therefore has higher infectivity, as established by recent studies.
The threat of COVID-19’s resurgence globally has created an urgent need to understand and develop strategies to counter the virus as soon as possible. The fact that the D614G strain is dominant wherever it has entered the population means that it has received much attention. The current study focuses on the energy reduction in terms of the configurational, single residue and mutational frustration associated with this substitution. In doing so, the researchers have found that the significant reduction in free energy and enhanced stability with a higher frequency of the ‘partly open’ conformation has caused the strain to become highly infective; more so, in fact, than the earlier strain.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources