Researchers from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) have conducted a study showing that the presence of anti-interferon-alpha (IFN-α) autoantibodies among patients with systemic lupus erythematosus (SLE) may predispose these individuals to more severe disease following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19).
The team says that SLE patients who developed COVID-19 had anti-IFN-α autoantibodies prior to infection, suggesting that the presence of these pre-existing autoantibodies might increase susceptibility to the virus.
Furthermore, SLE patients who had pre-existing autoantibodies were found to have higher rates of severe disease than those who did not.
“Our observations suggest that the presence of these autoantibodies may predispose SLE patients to infection with SARS-CoV-2 with a more severe presentation and represent an additional risk factor in this patient population,” write Sarthak Gupta and colleagues from the National Institute of Arthritis and Musculoskeletal and Skin Diseases in Bethesda, Maryland.
Anti-IFNα autoantibodies could therefore be a useful prognostic factor for predicting which patients with SLE might develop COVID-19 and for guiding clinical decisions about the management of these patients, adds the team.
A pre-print version of the paper is available on the server medRxiv*, while the article undergoes peer review.
Type 1 IFNs play a key role in the immune response to viral infection
Type 1 IFNs, including IFNα, play important roles in both the innate and adaptive immune responses and are essential in the host defense against viral infection.
Defects in type 1 IFN signaling pathways are known to result in immunodeficiency and it has been suggested that dysregulation in the type I IFN pathway plays a key role in the pathogenesis of the autoimmune condition SLE.
Furthermore, anti-type 1 IFN autoantibodies have been reported in patients with SLE and have also been associated with life-threatening COVID-19 among the general population.
Together, these observations led Gupta and colleagues to hypothesize that SLE patients who carried anti-IFNα autoantibodies before COVID-19 became pandemic in 2020, might be at a higher risk of developing the disease. They also proposed that the presence of these autoantibodies could help to guide management and prevention strategies.
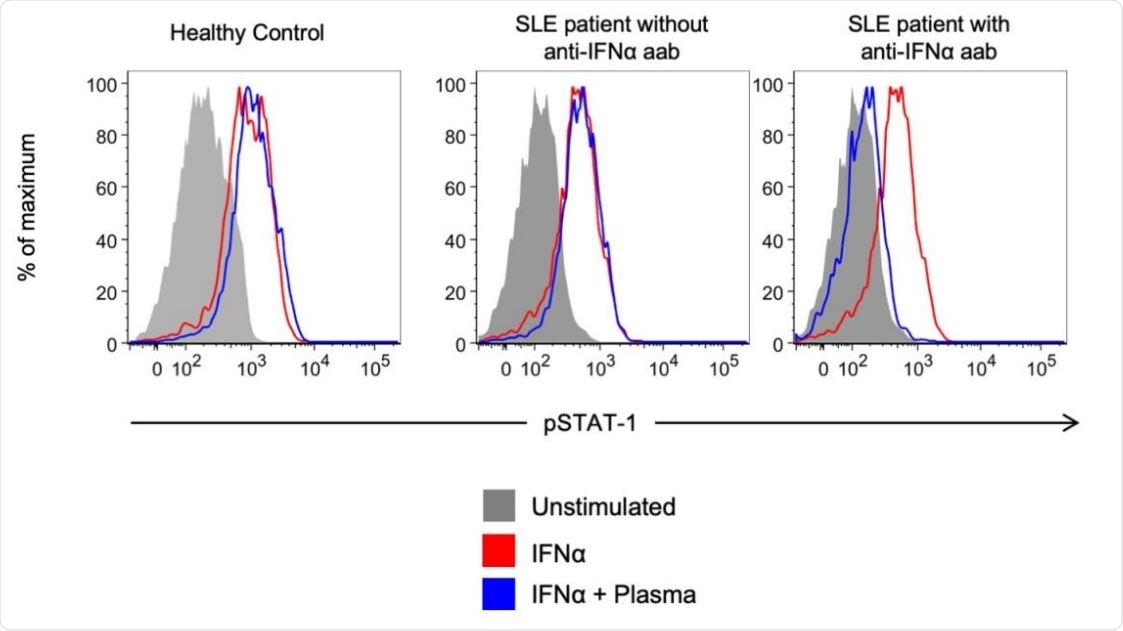
Representative example of detection of blocking anti-IFNα autoantibodies. Healthy control PBMCs were incubated with 10% plasma from healthy controls or from autoantibody- positive or negative SLE subjects with COVID-19, and then left unstimulated or stimulated with recombinant human IFNα. IFN-induced phosphorylation of STAT1 was measured by flow cytometry.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
What did the researchers do?
The team investigated whether the presence of anti-IFNα autoantibodies prior to 2020 was associated with COVID-19 in patients with SLE.
The researchers studied SLE patients (aged 26 to 57 years) who had developed COVID-19 between April 1st and October 1st, 2020.
Biobanked plasma samples that had been taken from these SLE patients and 119 age-matched healthy controls prior to 2020 were tested for anti-IFNa autoantibodies by enzyme-linked immunosorbent assay (ELISA).
To test whether these anti-IFNα autoantibodies could block IFNα signaling, the team used flow cytometry to see if they could block the signal transducer and activator of transcription 1 (STAT1) phosphorylation following stimulation with recombinant human IFNα (rhIFNα) in vitro.
What did the study find?
Of the ten SLE patients studied, seven patients had mild to moderate COVID-19 symptoms that were managed in the home setting and three had severe symptoms requiring hospitalization and oxygen supplementation.
Four of the ten SLE patients (40%) had anti-IFNα autoantibodies and six (5%) of the healthy controls also tested positive for the autoantibodies.
Longitudinal assessment of the SLE samples confirmed that the autoantibodies had been present for up to three years prior to the diagnosis of COVID-19 (as far back as 2017).
SLE patients with pre-existing anti-IFNα autoantibodies had more severe COVID-19
Patients with SLE who had pre-existing anti-IFNα autoantibodies had more severe symptoms of COVID-19.
For example, the rate of hospitalization was higher among those with anti-IFNα autoantibodies (2 out of 4) than among those who did not have the autoantibodies (1 out of 6).
Among the four SLE patients who were positive for the autoantibodies, plasma samples from two (50%) of them were able to block the activity of IFNα in vitro, through neutralization of rhIFNα induced STAT1 phosphorylation.
None of the samples from the six SLE patients who did not have the autoantibodies or from the six healthy controls who did have the autoantibodies were able to inhibit this STAT1 phosphorylation by rhIFNα.
What are the clinical implications of the findings?
The researchers say the findings suggest that the presence of ant-IFNα autoantibodies may predispose SLE patients to infection with SARS-CoV-2 that has a more severe presentation and may represent an additional risk factor among this patient population.
“Anti-IFNα autoantibodies may be pathogenic and could prove to be a helpful prognostic marker to predict which SLE patient may develop COVID-19 and inform preventive measures and management of this subset of patients,” concludes the team.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.