The race to find an effective vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has assumed a new urgency. The effort continues in light of an ongoing resurgence of the coronavirus disease 2019 (COVID-19) pandemic in several parts of the world, where the rates of new infection have spiked once again.
The SARS-CoV-2 spike protein’s receptor-binding region (RBD) is a prime target for vaccine development, as it is the crucial zone at which the virus engaged the host cell receptor angiotensin-converting enzyme 2 (ACE2), which in turn facilitates the subsequent membrane fusion that allows the virus to gain entry into and to infect the target cell. However, RBD subunits are not very immunogenic, hindering these efforts.
Some attempts to increase the immunogenicity include using multimers or attaching the RBD to a carrier to increase the antigen size. However, this may not only alter the RBD structure presented to the immune cells but may also result in a longer and more difficult production process.
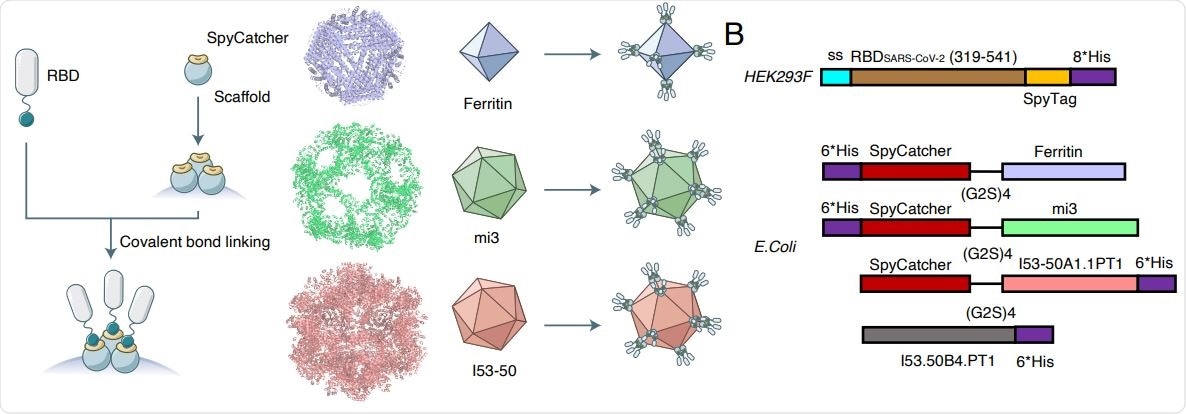
Construction and structural characteristics of RBD-conjugated nanoparticles. (A)Sketch of RBD nanoparticle design. The left flow diagram shows a brief introduction to the modification to RBD and nanoparticle scaffolds with fusion of SpyTag-SpyCatcher system. The right schema display ideal nanoparticles with full valency of RBD. Colors of each nanoparticle is accordant to the displayed palette of the following charts.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
RBD-Conjugated Nanoparticle Vaccine Candidates
The current study describes three vaccine candidates based on RBD-conjugated nanoparticles (NP), using covalent bonds in the well-established SpyTagSpyCatcher system. Here, the SpyTag is covalently linked to the C-terminus of the RBD, and the SpyCatcher to the NP. They thus fuse, linking the antigen to the NP scaffold. The conjugation was verified to leave the RBD structure essentially intact.
The three vaccine candidates used self-assembled ferritin NPs, mi3 NP protein, and 153-50 NPs, which form octahedral, dodecameric, and icosahedral particles to which the RBD is linked.
Increased Immunogenicity
In a mouse study, the adjuvanted monomeric RBD failed to produce detectable antibodies after the priming dose, but the binding antibody titer increased by 72 to 168 times following the priming dose of the RBD-NP conjugates. After 1 and 2 booster doses of the monomeric adjuvanted RBD and the conjugated RBD-NPs, the antibody titer increased significantly, but much higher responses were seen with the latter.
The ratio of IgG1 to IgG2 titers remained above 1 throughout, which indicates a Th2-biased immune response, and therefore a lower risk of antibody-dependent enhancement (ADE) of the disease.
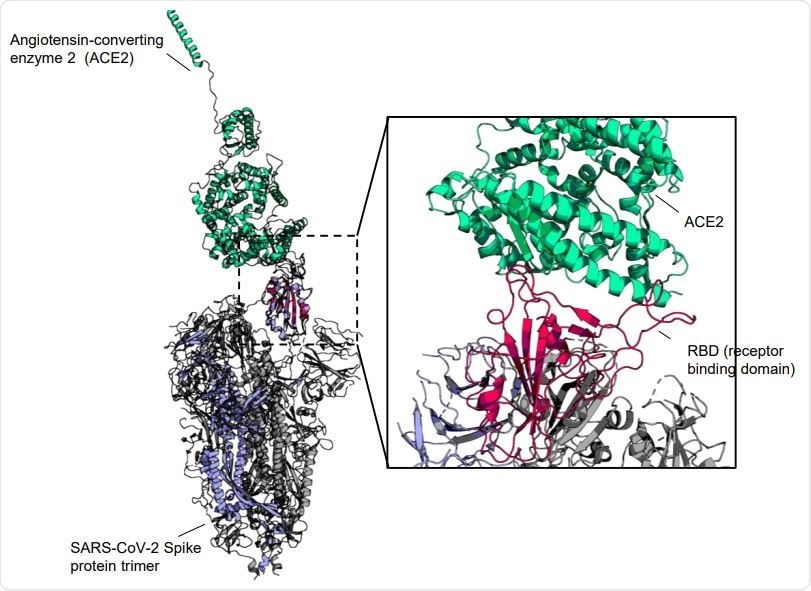
Co-structure of SARS-CoV-2 spike protein RBD with human ACE2. The SARS-CoV-2 spike protein trimer (Marine blue for chain with up-conformation RBD and grey with down-conformation RBD) (PDB code: 6VSB) is aligned to the complex of RBD (red) and human ACE2 (Light green) (PDB code: 6M0J) at the up-conformation RBD to display the binding interface.
Neutralizing Capacity up to 120-Fold Higher
The researchers found that these vaccines, produced 8-120-fold greater neutralization following the vaccination, compared to the monomeric RBD, when the serum from immunized mice was incubated with either the SARS-CoV-2 pseudovirus and the wildtype virus.
Secondly, these sera prevented RBD from attaching to ACE2 or to neutralizing antibodies in vitro. The binding to the antibody was much higher than with the SpyTag-RBD monomer. This may indicate the higher affinity of these RBD-conjugated NPs to the B-cell receptors specifically targeting the viral RBD. The stronger competitive inhibition offered by the RBD in the NP conjugates compared to the monomer suggests that the strength of inhibition is related to the number of copies of the RBD on the surface. Moreover, it may indicate that the spike protein (or S-protein) RBD of the virus is occupied
Thirdly, these vaccines are not only stable under a range of physical conditions, but their assembly is highly adaptable, which allows their manufacture to be rapidly scalable.
Conclusion
The investigators point out that these NP scaffolds could easily be linked to antigens other than the RBD from future pathogens, which would reduce the downtime required to understand the structure of the latter before being able to initiate vaccine development. This approach obviates the need to screen or express the antigen, select a suitable NP scaffold, assemble the particle, and confirm the immunogenicity of the particle.
This platform is also friendly to commercial manufacturers in that it yields a high amount of protein components for the vaccine, thus reducing the time required to bring forth a candidate vaccine.
The researchers say, “These results supported that our designed SARS-CoV-2 RBD-conjugated nanoparticle was a competitive vaccine candidate and the carrier nanoparticles could be adopted as a universal platform for future vaccine development.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Kang, Y.-F. et al. (2020). Rapid Development Of SARS-Cov-2 Receptor Binding Domain-Conjugated Nanoparticle Vaccine Candidate. medRxiv pre-print. doi: https://doi.org/10.1101/2020.11.03.366138, https://www.biorxiv.org/content/10.1101/2020.11.03.366138v1
- Peer reviewed and published scientific report.
Kang, Yin-Feng, Cong Sun, Zhen Zhuang, Run-Yu Yuan, Qingbing Zheng, Jiang-Ping Li, Ping-Ping Zhou, et al. 2021. “Rapid Development of SARS-CoV-2 Spike Protein Receptor-Binding Domain Self-Assembled Nanoparticle Vaccine Candidates.” ACS Nano 15 (2): 2738–52. https://doi.org/10.1021/acsnano.0c08379. https://pubs.acs.org/doi/10.1021/acsnano.0c08379.