Researchers in the United States have estimated that the proportion of people in New York City who developed antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the first wave of the coronavirus disease 2019 (COVID-19) pandemic is around one in eight.
Larry Luchsinger from New York Blood Center (NYBC) and colleagues from Regeneron Genetics Center, Imanis Life Sciences, and Regeneron Pharmaceuticals, Inc. say their findings suggest that background or “herd immunity” continues to be low and that eight months into the US pandemic, it is likely that the proportion of susceptible individuals remains very high, possibly greater than 80%.
The team says the study also suggests that the presence of antibody-based immunity is not necessarily associated with the development of neutralizing antibodies against SARS-CoV-2.
The researchers say the strategy they used to estimate seroprevalence in this study could be leveraged for future rapid assessment of seroprevalence/seroconversion in this cohort to help guide public health decision making.
A pre-print version of the paper is available on the server medRxiv*, while the article undergoes peer review.
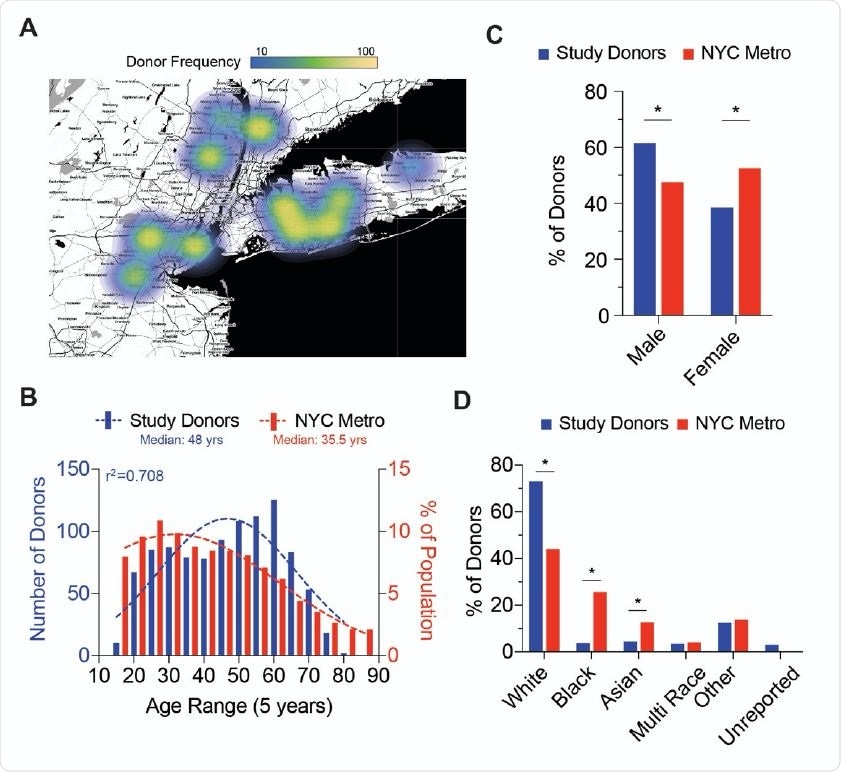
Blood Donor Demographics of NYC Metro Area A; Choropleth of donation site locations used for the collection of blood donor samples. Heatmap (gradient bar, top) indicates the frequency of donors collected per site. B; Distribution of NYC Metro area donor age (red bars) compared to NYC demographics (blue bars). Dotted lines represent the best fit to a Gaussian distribution and r2 value represents calculated goodness of fit to distribution plot. C; Gender frequency of NYC Metro area donors (red bars) compared to NYC demographics (blue bars). Chi-square test for goodness of fit to observed (donors) versus expected (NYC demographics) results; * p<0.01 D; Ethnicity frequency of NYC Metro area donors (red bars) compared to NYC demographics (blue bars). Chi-square test for goodness of fit to observed (donors) versus expected (NYC demographics) results; * p<0.01

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Serological tests of could be valuable for guiding public health policies
Since the first cases of SARS-CoV-2 were identified in Wuhan, China late December 2019, transmission models have suggested that recovery following infection may provide antibody-mediated immunity against future infection.
Serological tests that can identify individuals who have acquired antibodies against the virus (seroconversion) and the frequency of this seroconversion (seroprevalence) in a population could be valuable for guiding the development of public health policies.
As the infection number continues to rise and vaccine programs are introduced, serologic testing will be crucial for monitoring the development of herd immunity - the point where enough people have become immune, and person-to-person transmission is no longer likely.
However, the tests used for population-wide serological assessment must be reliable, credible, reproducible and high-throughput, say Luchsinger and team.
Furthermore, it is vital to understand the degree of correlation of any given assay’s “reactivity” with the presence of neutralizing antibodies, say the researchers, since only a subset of virus-specific antibodies exhibit neutralizing activity.
“Thus, studies that evaluate serological test designs are necessary to associate a serological result with a probability of immunity,” writes the team. “These data, then, can be used to assist public health officials in modeling projections and in informing policy-making decisions, including the safe ‘reopening’ of cities, states, and regions.”
A range of SARS-CoV-2 antibody assays are currently available, but differences in test characteristics, particularly sensitivity, could lead to variability and potential bias when estimating immunity levels among different locales or subpopulations.
However, two platforms that have been widely cited are in-house enzyme-linked immunosorbent assays (ELISA) and high-throughput serological assays (HTSA), say Luchsinger and team.
What did the current study involve?
New York City (NYC) was one of the first epicenters of the COVID-19 pandemic and had the highest case count per capita in the United States.
“Seroconversion, therefore, is likely to be substantial in a random sampling of NYC residents,” say the researchers.
The team conducted a point-in-time 1,000-person cohort study involving serial blood donors (aged a median of 48 years) in the NYC metropolitan area.
Plasma samples randomly collected from NYBC blood donation centers between June and July 2020 were tested using multiple commercially available assays, including ELISAs and HTSAs.
These were then tested and associated with assays for neutralizing antibodies.
What did the study find?
Among the 1,000 blood donor samples collected, the estimated seroprevalence rate using a test called the Ortho Clinical Diagnostics VITROS 120 Total Ig Test (Ortho) HTSA assay indicated a seroprevalence of 12.1%.
Another test called the Abbott Labs Architect SARS-CoV-2 IgG (Abbott) HTSA assay indicated that the seroprevalence was 10.9%.
The researchers also found that ELISA assays, which are the current gold-standard of serological quantification, corresponded with donor seropositivity as detected by these HTSAs, thereby validating the use of ELISAs in this context.
Importantly, the assays showed that the nature of the antibody-mediated immunity was not invariably associated with the development of neutralizing antibodies. Neutralizing activity against SARS-CoV-2 was wide-ranging and skewed towards slow-to-moderate titers of neutralizing antibodies.
The team says the data suggest that the seroconversion rate was around 1 in 8 among the blood donors since the pandemic began in NYC and that this estimate is in agreement with other reports from state and local departments of health.
What are the implications of the findings?
“Our findings demonstrate a comparable seroprevalence estimate can be discerned using a widely accessible blood donor population and it [is] an important metric during this catastrophic outbreak,” write the researchers.
This strategy can therefore be leveraged in its design for future studies to implement rapid seroconversion/seroprevalence monitoring, they say.
Furthermore, “these conclusions suggest that in the absence of a vaccine, “background” or “herd” immunity continues to be low at four months post commencement, and, now eight months into the US pandemic, it is probable that the susceptible population remains very high, and possibly at ~80% or greater,” the team warns.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Luchsinger L, et al. Seroprevalence of Anti-SARS-CoV-2 Antibodies in a Cohort of New York City Metro Blood Donors using Multiple SARS-CoV-2 Serological Assays: Implications for Controlling the Epidemic and Reopening. medRxiv, 2020. doi: https://doi.org/10.1101/2020.11.06.20220087, https://www.medrxiv.org/content/10.1101/2020.11.06.20220087v1
- Peer reviewed and published scientific report.
Jin, Daniel K., Daniel J. Nesbitt, Jenny Yang, Haidee Chen, Julie Horowitz, Marcus Jones, Rianna Vandergaast, et al. 2021. “Seroprevalence of Anti-SARS-CoV-2 Antibodies in a Cohort of New York City Metro Blood Donors Using Multiple SARS-CoV-2 Serological Assays: Implications for Controlling the Epidemic and ‘Reopening.’” Edited by Nicholas J. Mantis. PLOS ONE 16 (4): e0250319. https://doi.org/10.1371/journal.pone.0250319. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0250319.