Canadian researchers from McMaster University, Ontario, have pinpointed platelet activating immune complexes in coagulation disorders associated with coronavirus disease 2019 (COVID-19), the condition caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Their study titled, “Platelet Activating Immune Complexes Identified in COVID-19 Associated Coagulopathy,” has been released preprint server medRxiv*.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The SARS-CoV-2 agent that causes COVID-19 is known to be associated with coagulation disorders. Thrombosis is commonly seen in patients critically ill with COVID-19, and this has been termed as COVID-19 associated coagulopathy (CAC).
The features of CAC are similar to heparin-induced thrombocytopenia (HIT), showing features of mild thrombocytopenia (low platelet counts) and diffuse arterial and venous thrombosis.
HIT and thrombosis
HIT is a condition where prominent features of thrombocytopenia are seen; these are associated with heparin treatment and lead to thrombosis. It is said to be caused by IgG-specific antibodies that target platelet-factor 4 (PF4). These two form complexes called immune complexes (IC). These immune complexes, in turn, can activate the platelets mediated by FcγRIIA receptor. The immune complexes are not a result of IgG-specific anti-PF4/heparin antibodies in COVID-19, but are caused by endothelial cell activation.
COVID-19 and IC
Recent studies have shown that these immune complexes are also associated with severe COVID-19. This study was undertaken to see the association between platelet-activating ICs in CAC patients.
Study design
For this study, the team of researchers included blood samples from ten critically ill patients with COVID-19. The samples were analyzed at the McMaster Platelet Immunology Laboratory (MPIL). To compare against the critically ill patients, samples from eight convalescent COVID-19 positive patients were also taken. Of these, five belonged to HIT patients prior to the pandemic (HIT group). Seven of the samples were from health controls before the pandemic (HC group).
The researchers tested these samples for HIT, and collated other details from the patients to aid in their assessment. These included the gender of the patient, platelet counts (minimum), use of heparin, events related to thrombosis or abnormal coagulation, diagnosis at admission and outcome.
Using anti-PF4/heparin enzymatic immunoassay, the anti-PF4/heparin antibodies were detected in all samples. IgG, IgM, and IgA PF4-heparin antibodies were quantified. IgG-specific anti-PF4/heparin EIA was performed for positive patients. Functional platelet activation was tested using serotonin release assay (SRA) with heparin (0.1, 0.3, and 100 U/mL). IgG-, IgA- and IgM specific antibodies against the RBD (receptor-binding domain) and spike protein of SARS-CoV-2 virus were also tested.
To measure endothelial activation, von Willebrand Factor (vWF) antigen levels were assessed using chemiluminescent immunoassay. The team assessed the VWF activity using a special assay. They measured the ADAMTS13 metalloproteinase enzyme and also measured the anti-ADAMTS13 antibody in all patients. This was done to determine the changes in the vWF antigen levels associated with ADAMTS13 activity.
Findings
Overall results of the study were:
- Among the 6 CAC patients, platelet activation was seen using the serotonin release assay
- This platelet activation was inhibited when the FcγRIIA receptor was blocked, indicating an IgG-specific immune complex (IC)-mediated reaction.
- This platelet activation was independent of heparin; when heparin was administered at therapeutic and high doses, the activation, however, was inhibited
- These 6 CAC samples also were positive for IgG-specific antibodies against the receptor-binding domain (RBD) or the spike protein of the SARS-CoV-2 virus
- There was also significant endothelial cell activation in these samples, indicated by increased vWF antigen and activity. Moreover, patients with COVID-19 thrombosis also had raised vWF antigen and its activity. This is not associated, however, with inhibition or block of ADAMTS13.
- There was no indication of thrombotic thrombocytopenic purpura (TTP).
- None of the controls had anti-SARS-CoV-2 antibodies
- Convalescent plasma from non-critically ill COVID-19 patients did not activate platelets in the SRA, despite having high titers of anti-SARS-CoV-2 antibodies. This indicated that the antibodies alone are not capable of platelet activation.
- Endothelial cell activation was found to contribute to coagulopathy wrote the researchers
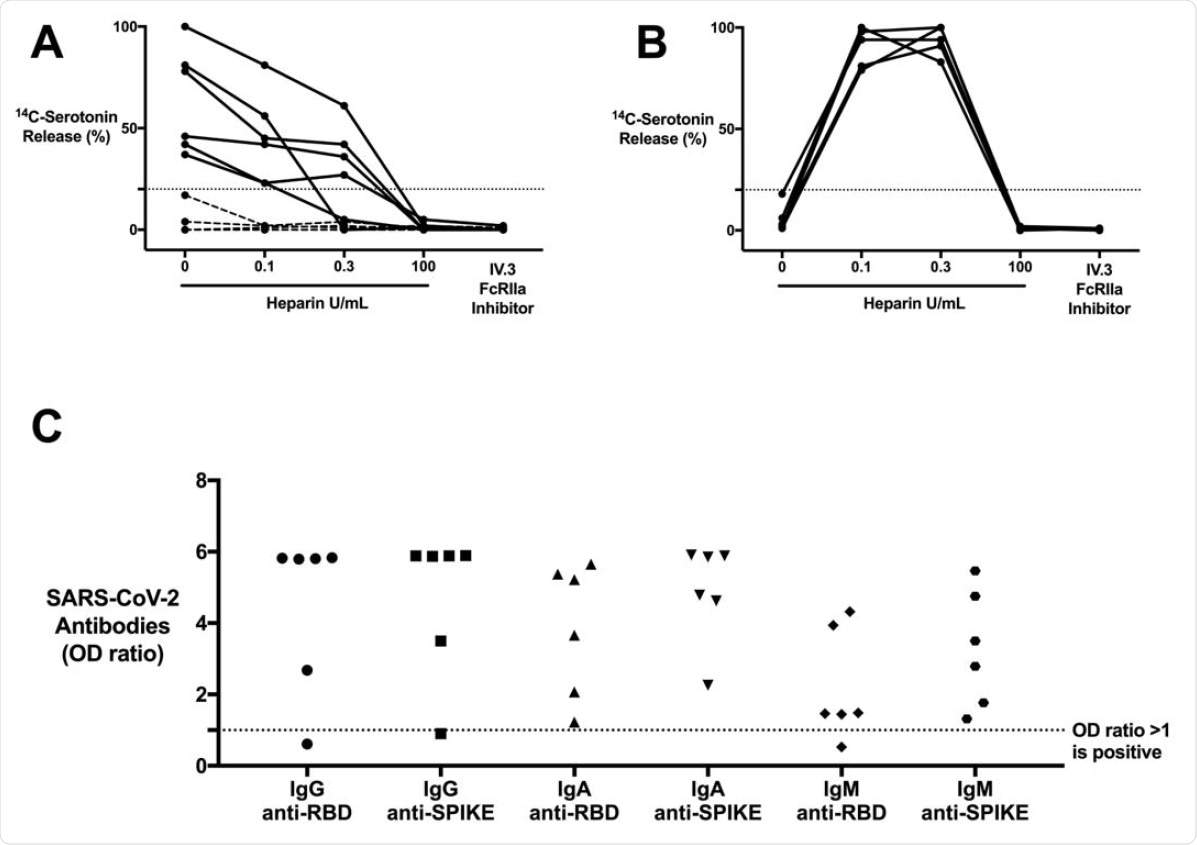
CAC patients with COVID-19 antibodies contain ICs that are capable of activating platelets in the SRA in a manner that is unique from HIT ICs. (A) CAC (n = 10) patient sera compared to (B) HIT patient (n = 5) sera, serving as a control, in the SRA. 14C-serotonin release was measured in the presence of increasing heparin doses or with addition of IV.3 (Fc?RIIA inhibitor). 14Cserotonin release >20% is positive in the SRA (horizontal dashed line). Most CAC patient sera (n = 6, solid line) demonstrate heparin-independent platelet activation, as opposed to classic HIT controls. Platelet activation was inhibited with IV.3 in both groups. (C) IgG, IgA, and IgM COVID-19 antibodies in platelet-activating CAC patient sera (n = 6). Antibodies were measured in the SARS-CoV-2 ELISA and include RBD and spike protein specificity. Values are shown as a ratio of observed optical density to the determined assay cut-off optical density. Values above 1 ratio are considered positive in the SARS-CoV-2 ELISA.
Conclusions and implications
The study provides evidence that immune complexes associated with COVID-19 were different from other severe coagulation disorders, including HIT and TTP. Thus COVID-19 patients with severe disease who develop thrombosis have immune complexes that activate platelets through FcγRIIA signaling. Patients with COVID-19 thrombosis also had raised vWF antigen levels and activity. However, the researchers did not associate this with inhibition or a block of ADAMTS13. The team wrote, “These ICs can produce a highly prothrombotic state resembling HIT but with unique platelet activating properties”.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Nazy, Ishac and Stefan D Jevtic, Jane C Moore, Angela Huynh, James W Smith, John G Kelton, Donald M Arnold (2020) Platelet Activating Immune Complexes Identified in COVID-19 Associated Coagulopathy. medRxiv. 2020.11.04.20226076; doi: https://doi.org/10.1101/2020.11.04.20226076, https://www.medrxiv.org/content/10.1101/2020.11.04.20226076v1
- Peer reviewed and published scientific report.
Nazy, Ishac, Stefan D. Jevtic, Jane C. Moore, Angela Huynh, James W. Smith, John G. Kelton, and Donald M. Arnold. 2021. “Platelet‐Activating Immune Complexes Identified in Critically Ill COVID‐19 Patients Suspected of Heparin‐Induced Thrombocytopenia.” Journal of Thrombosis and Haemostasis 19 (5): 1342–47. https://doi.org/10.1111/jth.15283. https://www.jthjournal.org/article/S1538-7836(22)00760-7/fulltext.