Researchers in France have conducted a study showing that people who are asymptomatic following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19) – elicit a polyfunctional antibody response to the virus.
The complement system refers to a group of proteins in the blood sera that enhances (complements) the ability of antibodies and other substances to destroy disease-causing pathogens.
The researchers performed cellular assays to compare the neutralizing, ADCC and complement activities across sera taken from people with SARS-CoV-2 infection who were asymptomatic, mildly symptomatic, or requiring hospitalization due to the development of COVID-19.
The team – from the Pasteur Institute in Paris and Paris-Est Créteil University – aimed to assess the extent and quality of the antiviral humoral response across the different disease severities.
Overall, the results indicated that asymptomatic infection induces a polyfunctional antibody response, a finding that the researchers say warrants further investigation in the evaluation of vaccine candidates and the study of COVID-19 immunopathology.
A pre-print version of the paper is available on the server medRxiv*, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The immune response to SARS-CoV-2 is the subject of intense investigation
Since the first cases of SARS-CoV-2 were identified in Wuhan, China, late last year (2019), the unprecedented spread of the virus has overwhelmed health care services across many parts of the globe.
In the absence of any vaccine or effective therapies, many governments implemented non-pharmaceutical interventions in an effort to control transmission, including lockdown and social distancing policies.
As these restrictions were relaxed, new epidemic waves occurred in many countries, demonstrating the need for “herd” or population immunity, whether acquired through exposure to infection or vaccination.
“Therefore, the immune response induced by SARS-CoV-2 is under intense investigation, aiming at informing vaccine design, identifying correlates of protection and determining the duration of protective immunity,” writes Timothée Bruel (Pasteur Institute) and colleagues.
Clinical outcomes vary widely between individuals
The clinical manifestations of COVID-19 are highly variable, ranging from asymptomatic or mild infection to severe disease, organ failure and death. Around one-half of infected individuals are asymptomatic, and little is known about the extent and quality of the antiviral immune response in this group.
Severe disease arises as a result of immunological dysfunctions, including increased inflammation and activation of the complement system.
Mild and asymptomatic infection triggers seroconversion and the production of neutralizing antibodies, although levels of these antibodies are lower in asymptomatic individuals.
“Whether such responses are protective is unknown,” writes the team.
The significance of the viral spike protein
The main surface structure SARS-CoV-2 uses to bind to and enter host cells is the spike glycoprotein, which attaches to the human receptor angiotensin-converting enzyme 2.
This spike protein is sensitive to antibody targeting, but the antiviral activity of these antibodies is not limited to neutralization; other mechanisms include antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
Among critically ill patients, antibodies form immune complexes that can trigger the activation of natural killer (NK) cells and complement deposition.
“However, whether such polyfunctional antibodies are induced during asymptomatic COVID-19 disease and whether they are able to eliminate infected cells remains unknown,” say Bruel and colleagues. “A deeper understanding of the immune response after asymptomatic SARS-CoV-2 infection is needed.”
What did the researchers do?
The team measured the anti-SARS-CoV-2 humoral immune response among SARS-CoV-2-infected individuals, of whom 52 were asymptomatic, 119 had mild disease, and 21 had severe disease that required hospitalization.
The researchers designed cellular assays to assess the ability of anti-SARS-CoV-2 antibodies to trigger complement deposition and to eliminate infected cells through NK-dependent ADCC.
This panel of assays allowed the team to establish a profile of the antiviral properties of antibodies across the different categories of disease severity.
“We focused our analysis on asymptomatic individuals since their humoral response remains poorly characterized, and compared them to mildly symptomatic and hospitalized patients.”
What did they find?
Sera from asymptomatic individuals elicited a polyfunctional antibody response that neutralized the virus, activated ADCC, and triggered complement deposition.
Antibody titers and antibody activities were slightly lower among asymptomatic individuals, resulting in reduced neutralization, although the differences were modest, say the researchers.
The neutralizing activity of anti-Spike antibodies correlated with their ability to induce ADCC ad complement deposition, regardless of the severity of disease.
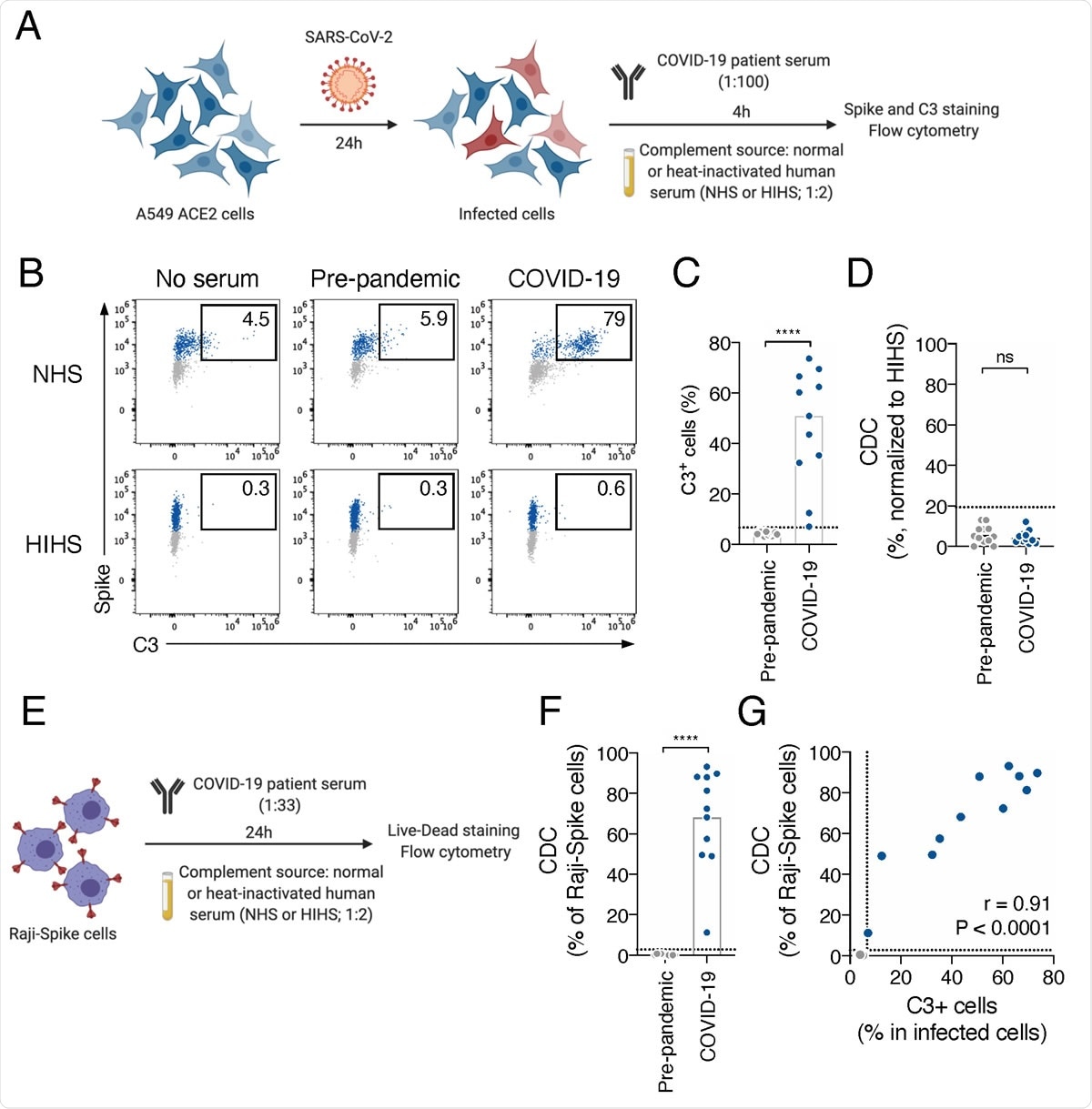
COVID-19 sera activate the complement. A. Schematic of the complement activation test on infected cells. A549-ACE2 cells are infected at a multiplicity of infection (MOI) of 1. 24 hours later, serum from pre-pandemic or COVID-19 patient is added (dilution 1:100) as a source of antibodies. Normal human serum (NHS) from a healthy donor is used as a source of complement and heat-inactivated human serum (HIHS) as control. 4 hours later, cells are stained with an anti-Spike and an anti-C3b/iC3b antibody. Complement deposition on infected cells is measured by flow cytometry. Complement-dependent cytotoxicity (CDC) induced by serum is also measured as the relative disappearance of infected cells compared to the “HIHS” condition. B. Complement deposition on infected cells was measured after culture with or without control or a COVID-19 serum in presence of NHS or HIHS. Results from a representative experiment are shown. Percentages indicate the proportion of C3+ cells among infected (Spike+) cells. C. Complement deposition with pre-pandemic (n=12) and COVID-19 patients’ (n=11) sera. The percentage of C3+ cells among infected cells is represented. Each dot represents the mean of 3 independent experiments for one serum donor. D. Complement-dependent cytotoxicity (CDC) of infected A549-ACE2 cells was calculated as the relative disappearance of Spike+ cells in the NHS compared to HIHS condition, with pre-pandemic (n=12) and COVID-19 patients’ (n=11) sera. Each dot represents the mean of 3 independent experiments for one serum donor. E. Schematic of the complement activation test on Raji-Spike cells. Raji-Spike cells are incubated with serum (heat-inactivated) from a pre-pandemic or a COVID-19 patient (dilution 1:100) and normal human serum (NHS) or heat-inactivated human serum (HIHS) as control (dilution 1:2). Complement-dependent cytotoxicity (CDC) induced by a serum is calculated as the relative cell death compared to the “no antibody” condition. F. Raji-Spike cells were cultured with sera from pre-pandemic individuals (n=11) or COVID-19 patients (n=11) and serum from a healthy individual as a source of complement. CDC was measured as the relative cell death compared to the no antibody condition. Each dot represents a different serum. G. Correlation of the C3 deposition on A549-ACE2 infected cells and CDC of Raji-Spike cells induced by sera from pre-pandemic individuals (grey, n=12) and COVID-19 patients (blue, n=11). In each graph, the dotted line corresponds to the positivity threshold calculated with pre-pandemic sera. In C, D and F, the bar indicate the mean and a Mann-Whitney test was performed (ns. = not significant, ****p<0.0001). In G, a Spearman correlation test was performed and the correlation r and p-value are indicated.
“Our results warrant further analysis”
“We show here that asymptomatic individuals mount a humoral immune response only slightly decreased when compared to symptomatic SARS-CoV-2 infections. In addition to neutralization, this response includes the ability to trigger ADCC and complement deposition,” say Bruel and team.
The researchers point out that non-neutralizing antibodies contribute to the protection provided by experimental influenza and HIV vaccines.
“It will be of interest to assess whether SARS-CoV-2 vaccine candidates elicit non-neutralizing antibody functions and whether this correlates with vaccine efficacy,” they write.
“Our results warrant further analysis of neutralization and other antibody functions in the evaluation of vaccine candidates and the study of COVID-19 immunopathology,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Bruel T, et al. Asymptomatic and symptomatic SARS-CoV-2 infections elicit polyfunctional antibodies. medRxiv, 2020. doi: https://doi.org/10.1101/2020.11.12.20230508, https://www.medrxiv.org/content/10.1101/2020.11.12.20230508v1
- Peer reviewed and published scientific report.
Dufloo, Jérémy, Ludivine Grzelak, Isabelle Staropoli, Yoann Madec, Laura Tondeur, François Anna, Stéphane Pelleau, et al. 2021. “Asymptomatic and Symptomatic SARS-CoV-2 Infections Elicit Polyfunctional Antibodies.” Cell Reports Medicine 2 (5): 100275. https://doi.org/10.1016/j.xcrm.2021.100275. https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(21)00103-8.