As the coronavirus global health crisis continues, detecting the presence of serum antibodies to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent that causes the coronavirus disease 2019 (COVID-19), is crucial. In doing so, scientists can gain a better insight into both the scale of the virus's spread and a population's resistance to it after initial infection.
Serum antibodies reflect prior exposure to the virus, and some may confer protection or immunity against subsequent reinfection.
In a recent study, a team of researchers at Northwestern University, USA, had found that nearly 20 percent seroprevalence in the United States metropolitan site, Chicago, over the summer, during a period when lockdown restrictions were eased.
They found that three-quarters of seropositive individuals retained detectable antibodies for at least 120 days or four months.
The new finding supports previous studies showing people who recovered from COVID-19 developed antibodies to protect against a second bout.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
A preprint article of the research group's findings is available on the medRxiv* server.
The role of serum antibodies
Antibodies are produced over days to weeks after infection with the virus. An antibody response's strength depends on many factors, including nutritional status, age, certain medications, being immunocompromised and illness severity.
Antibodies are detected in the blood of people who are tested after an infection. When antibodies are present in the blood, it means that the body has generated an immune response to the infection.
A COVID-19 antibody test, known as a serology test, is a blood test that can detect if a person has developed antibodies to SARS-CoV-2. Being exposed to the virus increases antibody serum levels.
Understanding the role of antibodies and the importance of serum antibody tests can help health experts estimate the virus's extent.
The study findings
To arrive at the study findings, the researchers used dried blood spots (DBS) that are easily collected at home using a simple finger prick method.
The team had previously described the development and sensitivity of a DBS test to measure immunoglobulin G (IgG) to the receptor-binding domain of the SARS-CoV-protein using a laboratory-based enzyme-linked immunosorbent assay (ELISA).
The participant base included a mix of people who were split into two categories. The first group was categorized as "essential," which meant they needed to work outside of their homes during the shelter-in-placer order; the second, "non-essential," were those who worked from home during the same period.
The researchers recruited the participants through two methods. First, community-based participants were recruited from ten zip codes in Chicago through social media advertising and news articles. Second, employees, students and faculty members from the Northwestern University Feinberg School of Medicine in Chicago were sent an email describing the study with a link to the website.
The participants were asked to complete a questionnaire about general health status and COVID-19 symptoms. The team provided the community participants with materials for DBS collection who returned their samples using a courier service provided by the research team.
Both Chicago and other places within Illinois were under a stay-at-home order from March 21 until May 30.
The researchers started sampling in late June 2020, a period when lockdown orders were lifted. The collection of samples went on through September 6.
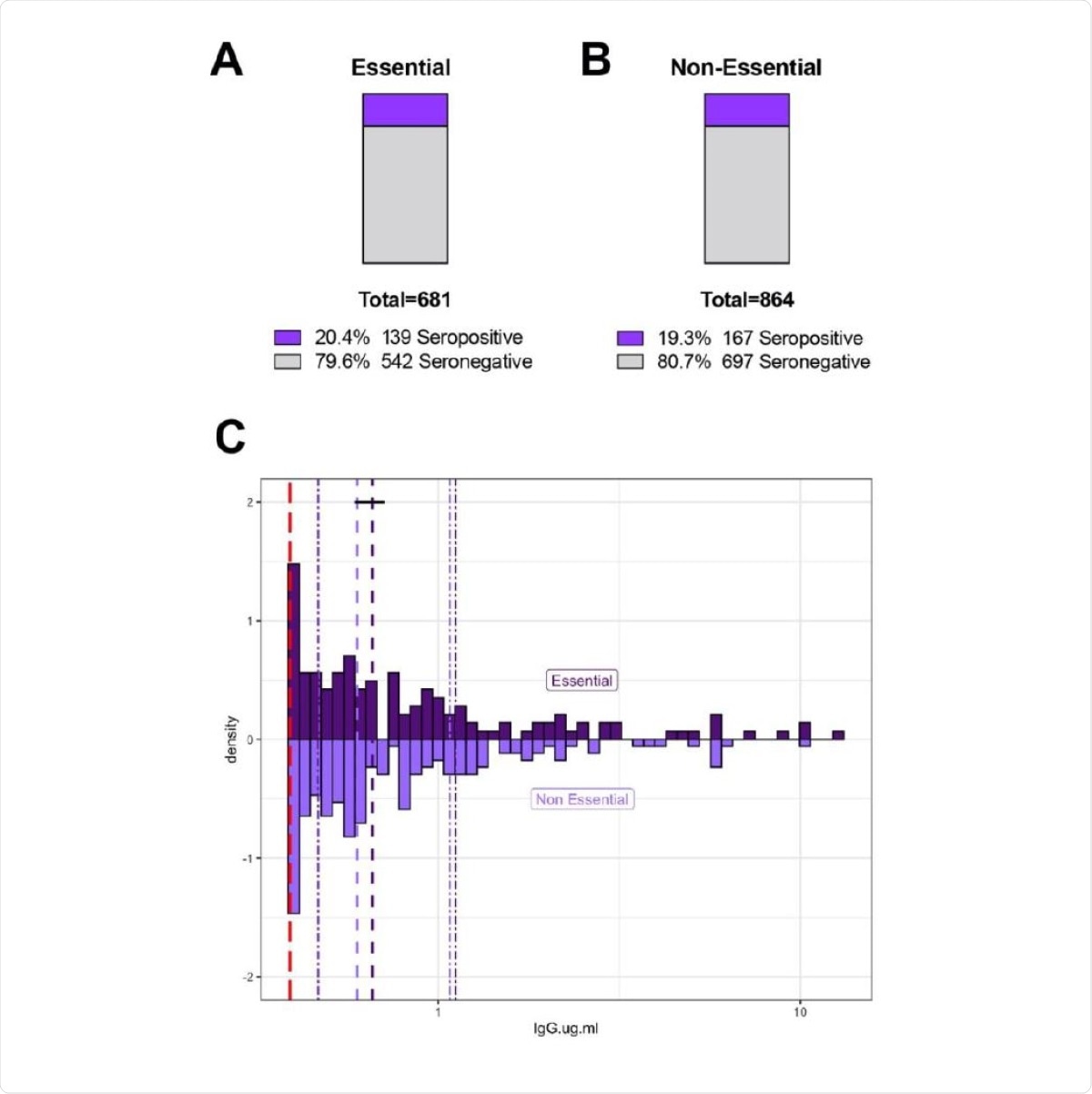
Similar rates of SARS-CoV-2 RBD IgG seropositivity between essential and non-essential workers. Fifteen hundred and forty-five unique SCAN community-acquired samples was acquired between June 24 - September 6, 2020 in the Chicagoland area. Participants self-reported essential worker status during the months of March-September; defined as leaving the residence for work and interacting with co-workers / public. A and B) Essential and non-essential reported groups have similar percent seropositivity at 20.4 % and 19.3%, respectively. C) Essential (n=139) and non-essential (n=167) groups have similar distributions of SARS-CoV-2 RBD IgG seropositivity with a median of 0.65 µg/ml and 0.59 µg/ml, respectively. The SARS-CoV-2 RBD IgG ELISA positivity threshold is denoted with the red dotted line at 0.39 µg/ml. Dashed purple lines represent quartiles. Statistics: Two-sample Kolmogorov-Smirnov test p=0.81.
Overall, the researchers found that the seroprevalence of IgG was 19.8 percent, with similar seroprevalence among samples obtained from the mail and those through an onsite DBS kit distribution.
Further, the team also noted no difference between seroprevalence in people who were known as "essential" and those who are "non-essential."
"These data underscore the importance of a self-collected, quantitative assay with adequate sensitivity to detect antibodies at the lower levels among non-hospitalized persons with community-acquired exposure to COVID-19," the researchers concluded in the study.
COVID-19 spread
The coronavirus pandemic continues to spread across the globe, with over 56.49 million cases confirmed. The virus has already claimed more than 1.35 million lives.
The United States reports the highest number of confirmed cases, reaching 11.55 million. India, Brazil, France, and Russia record increasing numbers of also, with over 8.95 million, 5.94 million, 2.11 million, and 1.99 million cases confirmed, respectively.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references:
- Preliminary scientific report.
Demonbreun, A., McDade, T., Pesce, L., et al. (2020). Patterns and persistence of SARS-CoV-2 IgG antibodies in a US metropolitan site. medRxiv. doi: https://doi.org/10.1101/2020.11.17.20233452, https://www.medrxiv.org/content/10.1101/2020.11.17.20233452v1
- Peer reviewed and published scientific report.
Demonbreun, Alexis R., Thomas W. McDade, Lorenzo Pesce, Lauren A. Vaught, Nina L. Reiser, Elena Bogdanovic, Matthew P. Velez, et al. 2021. “Patterns and Persistence of SARS-CoV-2 IgG Antibodies in Chicago to Monitor COVID-19 Exposure.” JCI Insight 6 (9). https://doi.org/10.1172/jci.insight.146148. https://insight.jci.org/articles/view/146148.