A large amount of evidence has accumulated that the reported cases of coronavirus disease 2019 (COVID-19) globally, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen, is significantly lower than the reality. A startling new case study of Chicago, led by a team of researchers at Northwestern University in Illinois, USA, suggests a 16-fold higher incidence of SARS-CoV-2 infection than previously thought. This is judging from seroprevalence (antibody presence blood testing) figures, rather than the number of RT-PCR-positives reported there (via swab sample testing of symptomatic patients).
The study underscores the importance of using a convenient and sensitive self-collected sampling method that can offer a quantitative assessment of the antibody titers in the community, even with low titers.
Serologic testing for antibodies is important in many ways in the current COVID-19 pandemic. Not only does it help us assess the scale of a population’s exposure to the pathogen, but it may also allow us to gauge levels of immunity through understanding the prevalence and titers of antibodies in the community. However, the testing method and the viral antigen against which antibodies are raised may vary, and with them, the seropositivity estimates.

Chicago - April 4, 2020: Chicago theater building with a man wearing a mask walking by during the coronavirus pandemic. Image Credit: iwonder TV / Shutterstock.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The team reported their findings on the preprint server medRxiv* in November 2020.
Dried blood spot serology
Currently, the majority of antibody tests are based on blood or serum testing. This, however, depends on the availability of a healthcare center and a skilled worker to administer them. Such studies are thus likely to be biased from the beginning. Another option is using point-of-care assays based on lateral flow devices, but these are neither as sensitive or as specific as laboratory assays.
The current Screening for Coronavirus Antibodies in Neighborhood (SCAN) study assessed seroprevalence as measured from dried blood spots (DBS) from a fingerprick. This method had been validated earlier for the qualitative measurement of anti-RBD IgGs using ELISA.
.jpg)
Dry blood spots on a collection card for COVID-19 IgG/IgM antibody screening. Image Credit: Marc Bruxelle / Shutterstock
The researchers used a dried blood spot (DBS) assay to quantitatively measure the presence of anti-spike receptor-binding domain (RBD) antibodies against SARS-CoV-2 in over 1,500 individuals. The participants were enrolled by online advertisements (813) or from a medical school (730 staff, faculty and non-treating staff).
The latter group included both essential workers (those working outside of the home) and non-essential workers (those working from home) during the initial lockdown of early 2020. The blood spots were on a card format, and could be either mailed back or dropped off at the testing center in person.
20% Seropositivity
The researchers found that about one in five of the tested samples showed anti-RBD IgG antibodies. The seropositivity was about 23% among those aged 18-29 years and 40-49 years, around 18-19% in those aged 30-39 years and 50-59 years. However, it was lowest, at around 13%, among those over the age of 60.
The proportion of detection was similar among samples sent in by mail or returned in person. The seroprevalence was also similar among essential and non-essential workers and among males and females.
The testing began after a partial relaxation of the stay-at-home orders, from late June 2020 through the first week of September 2020. The importance of the comparable seropositivity estimate among essential versus non-essential workers could be because of the late timing of the survey, when some shelter-in-place orders had already been relaxed.
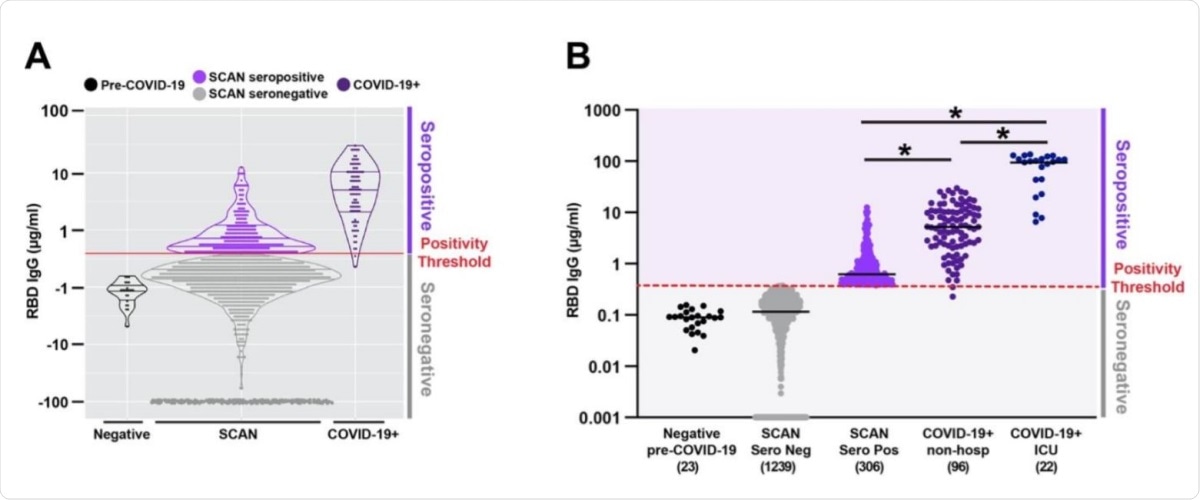
Quantitative measure of IgG directed to the receptor binding domain of SARSCoV-2 spike glycoprotein. Samples were acquired through the Screening for Coronavirus Antibodies in Neighborhood (SCAN) between June 24, 2020 and September 6, 2020 (n= 1545). A) Overlap between the IgG range seen in the community-acquired SCAN seropositive samples (light purple) and non-hospitalized COVID-19+ seropositive samples (dark purple, far right). B) 19.8% (306 of 1545) of SCAN samples were seropositive with a median IgG concentration of 0.62 µg/ml SCAN for the seropositive group. The median concentration of the seronegative SCAN group was 0.11 µg/ml. As a comparator, shown is the range of IgG detected from 96 non-hospitalized and 22 ICU hospitalized individuals with COVID-19 documented by a positive nucleic acid test for SARS-CoV-2 virus. The median IgG concentration was 5.2 µg/ml for the non-hospitalized COVID-19+ group and 98.5 µg/ml for the ICU hospitalized COVID-19+ group. The SARS-CoV-2 RBD IgG ELISA seropositive threshold is marked by the red line at 0.39µg/ml. Two hundred and forty-four seronegative samples with an IgG concentration below 0.001 were plotted at 0.001. Comparing seropositive groups * p<0.0001 by Wilcoxon-Mann-Whitney Test. Both seronegative groups are significantly different than all seropositive groups.
IgG titers comparable in mild and asymptomatic cases
The researchers also observed similar IgG titers among those who were seropositive in the study, and those who were identified by self-detection but not hospitalized. This could indicate that the degree of immune stimulation and immune response was similar among these groups.
However, the median titer among those admitted to an intensive care unit was 12 times higher than the latter group (98.5 μg/ml versus 5.2 μg/ml).
On the other hand, only 19 participants (1.2%) reported that they had been tested positive for COVID-19 before this date. Of these, 18 were also anti-RBD IgG positive.
Anti-RBD antibodies more useful than anti-nucleocapsid antibodies
The researchers cross-checked 28 samples from patients who had recovered after symptomatic COVID-19, all of whom had a positive viral test, against 92 samples from symptomatic individuals who did not have such a result. They found both groups showed a low level of agreement between antibodies to these two antigens, nucleocapsid (N) and RBD
About a fifth of the 28 symptomatic COVID-19 positives had only anti-RBD IgG without anti-N antibodies. Conversely, around 75% were positive for both antibodies. Only one participant was negative for both antibodies.
Of the 92 SCAN samples, over 70% had anti-RBD IgG, among which 45/65 had only anti-RBD IgG, while 20/65 samples had both antibodies, and none had both negative. The implication is that antibodies targeting the RBD are probably more useful in detecting the seroprevalence.
Most of the participants who had an initial seropositive test continued to show detectable median concentrations of IgG from day 0 to 73-166 days later, that is, for about 4 months. In one case, the anti-RBD IgG titer was stable for 3 months and then spiked. This patient had a history of suggestive symptoms two days before, but the PCR test was negative. However, the test was done 25 days after the earliest symptom, which may account for the negative result. Or, the viral load may never have crossed the limit of detection of the test. However, the researchers attribute this result to possible re-exposure to the virus.
What are the implications?
The researchers detected a high prevalence of anti-RBD antibodies, but it is not clear if they are protective or neutralizing. Further studies will be required to clarify if seropositives are less susceptible to infection than seronegatives.
About 75% of the community showed persistent seropositivity, with at least one showing some evidence of re-exposure. With respect to the longitudinal pattern of anti-RBD IgG, the researchers say: “We hypothesize this pattern reflects re-exposure to SARS-CoV-2. Whether this pattern portends what might occur with re-exposure generally, or even after vaccines become available, remains for future researchers to explore.”
The study concludes: “These data highlight the importance of quantitative, self-collected seroprevalence in monitoring the COVID-19 pandemic response. The SCAN platform, which relies on simple at home monitoring combined with laboratory precision, is positioned to help address this, and other, knowledge gaps.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources