Studies on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of the ongoing COVID-19 pandemic, have established that the spike protein of the virus is key to infection. The receptor-binding domain (RBD) of the spike protein binds to the human angiotensin-converting enzyme 2 (ACE2), followed by membrane fusion with the host cell membrane and viral entry.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
In response to virus infection, the host produces specific immunoglobulin (IgG) antibodies to the spike protein, RBD, and nucleoprotein, starting between six to 15 days after infection. Antibodies to the spike protein and RBD antigens are correlated with T cell response and viral neutralization in in vitro studies. However, it is unclear if reinfection can occur in patients who have mounted an immune response, although some reinfection cases have been reported. Hence, there is a need to understand and characterize the immune response after SARS-CoV-2 infection.
IgG and neutralizing antibodies to SARS-CoV – the betacoronavirus responsible for the 2002 SARS outbreak – have been known to persist for 2–3 years after infection, with recent reports showing persistence even up to 12–17 years. After MERS infection, antibodies have been seen for 34 months after infection. However, longitudinal studies of SARS-CoV-2 antibodies are limited for many reasons, including the shortness of present studies’ durations and low sample numbers.
Determining antibody dynamics over time
In a new study published on the medRxiv* preprint server, a team of UK-based researchers have reported on antibody kinetics and persistence after SARS-CoV-2 infection. In their ‘COVID-19 Staff Testing of Antibody Responses Study’ (Co-STARS) undertaken between April and October 2020, the team enrolled 3,555 UK health care workers, of which 349 were seropositive for SARS-CoV-2. The positive participants were followed for up to 7 months after symptom onset, providing 1,163 monthly serological samples. The team measured serum antibodies using MSD Chemiluminescent binding assay, while the detection of SARS-CoV-2 was done using real-time polymerase chain reaction reverse transcription (RT-PCR).
Alongside the experimental tests, the team also statistically analyzed the data and antibody dynamics, which were estimated using two gamma models to include an assumption for exponential decay of antibodies and another for a long-lived plateau of antibodies after infection.
The authors observed a faster decay in the nucleoprotein antibody compared to those of the spike protein and RBD antibodies. After 200 days of symptom onset, 75% of the participants were positive for the nucleoprotein antibodies, while 99% were still positive for the spike protein antibodies.
By modeling the data obtained, the authors estimate that 95% of people after SARS-CoV-2 infection will have spike protein antibodies until 465 days after symptom onset. This was under the most stringent assumption that spike protein antibodies decay exponentially. Under more optimistic assumptions, antibodies are predicted to remain for a long time.
Weekly measurements of the antibodies showed they increased rapidly in the first three weeks and maintained high levels four to 10 weeks after symptom onset, with peak antibody levels observed 30–40 days after symptoms developed.
The team's analysis showed that the half-lives for the nucleoprotein, RBD, and spike protein antibodies were 60 days, 102 days, and 126 days, respectively, under the gamma decay model, which assumes exponential decay of antibodies. The half-lives for the RBD and spike protein antibodies were increased to 110 days and 364 days under the gamma plateau model, which assumes long-lived antibodies.
A sigmoidal relationship between the antibody binding titers and percentage binding or ACE2 receptor blocking for the spike protein and RBD antibodies indicated that above an antibody threshold titer, there is a significant increase in binding/ACE2 receptor blocking.
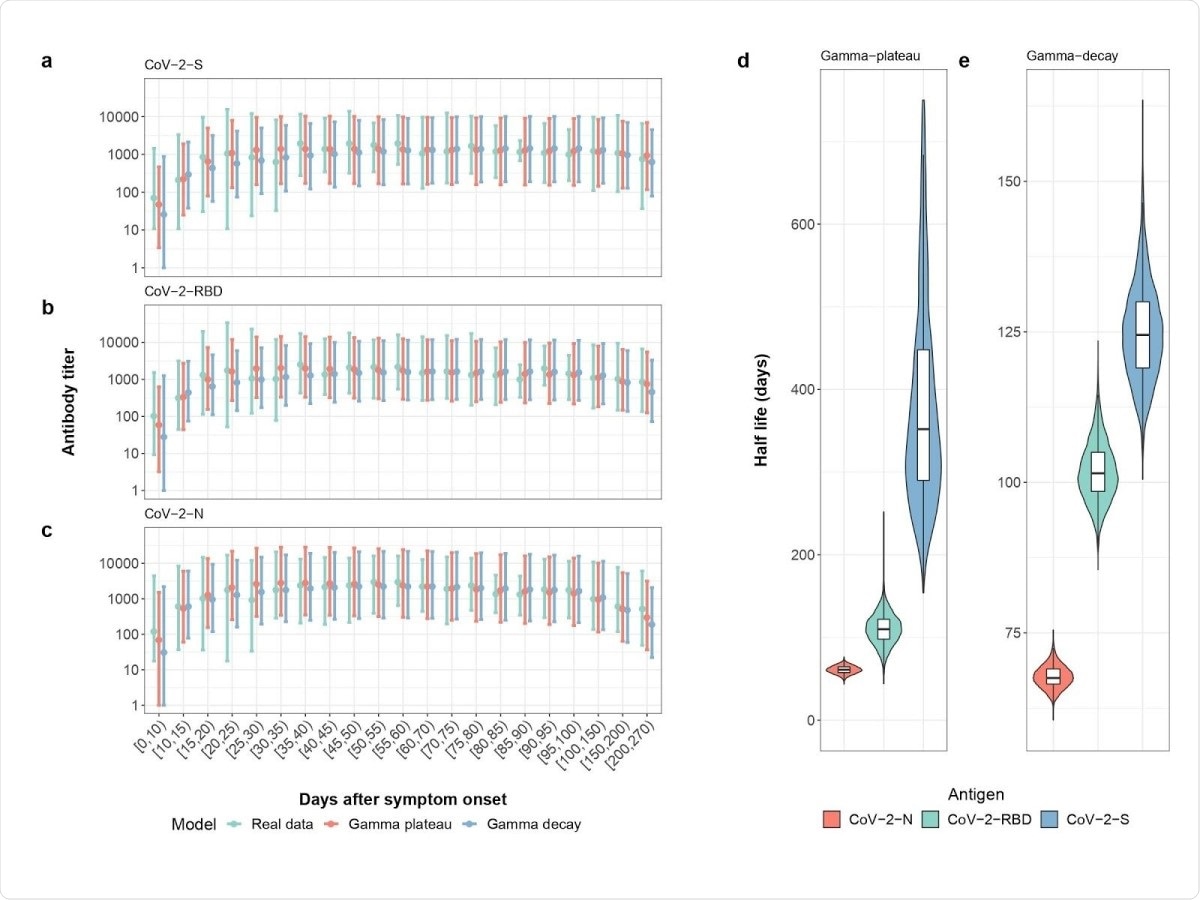
Measured and Modeled Weekly Mean Antibody Titer . Real data (green), gamma-plateau model (red), gamma-decay model (blue) for a) the spike antibody, b) the RBD antibody and c) the N-antibody. Modeled half-lives of antibody decay for d) The gamma-plateau model and e) gamma-decay model. Colors represent the three different antibodies tested: those for the spike protein (blue), nucleocapsid protein (red), and receptor-binding domain (green).
Implications of antibody levels detected
Although the duration of antibodies persisting in infected individuals conflicts with some other results showing rapid decay of antibodies, the results are similar to those observed for SARS-CoV and MERS.
The lower duration of the presence of nucleoprotein antibodies is important to take into account when designing diagnostic tests and seroprevalence-based public health interventions. Since none of the positive participants in the study needed hospitalization, the authors write, the participants were a representative sample of COVID-19 patients. This is because the majority of COVID-19 cases do not need hospitalization.
Although there is no definitive understanding of the protection to SARS-CoV-2 offered by antibodies as yet, animal studies support the idea that neutralizing antibodies correlate to viral immunity. But, it still remains to be seen if the level of antibodies detected are enough to provide long-term immunity from SARS-CoV-2. Neutralization assays after a year of infection will be needed to determine this.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Grandjean, L. et al. (2020) Long-Term Persistence of Spike Antibody and Predictive Modeling of Antibody Dynamics Following Infection with SARS-CoV-2. medRxiv. https://doi.org/10.1101/2020.11.20.20235697, https://www.medrxiv.org/content/10.1101/2020.11.20.20235697v1
- Peer reviewed and published scientific report.
Imai, Kazuo, Yutaro Kitagawa, Sakiko Tabata, Katsumi Kubota, Mayu Nagura‐Ikeda, Masaru Matsuoka, Kazuyasu Miyoshi, et al. 2021. “Antibody Response Patterns in COVID‐19 Patients with Different Levels of Disease Severity in Japan.” Journal of Medical Virology 93 (5): 3211–18. https://doi.org/10.1002/jmv.26899. https://onlinelibrary.wiley.com/doi/10.1002/jmv.26899.