The coronavirus disease 2019 (COVID-19) pandemic has now spread for over 10 months, evading capture by effective antivirals. The earliest vaccines are still in phase III of their clinical trials. Remdesivir is among the very few drugs that show even moderate benefit in this illness.
_-_1200_width.jpg)
This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Image Credit: NIAID / Flickr
The virus causing COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a betacoronavirus that is thought to have leaped the species barrier to infect humans. Many believe the virus originated in horseshoe bats through an unidentified intermediary host.
This virus possesses several structural and nonstructural proteins (nsps). These include the primary spike (S) antigen, the nucleocapsid (N) antigen, and replication-essential nsps. The spike glycoprotein mediates cell entry via its engagement with angiotensin-converting enzyme 2 (ACE2) receptors on the target cell, leading to cleavage of the spike protein and internalization of the virus by endocytosis.
At this point, the N protein breaks down by the action of the cell proteases, freeing the viral single-stranded RNA, which undergoes replication and translation to produce both further copies of the viral genome and the viral proteins necessary for both replication and viral assembly to form infectious viral particles. This happens within the replication-transcription complex (RTC). The outcome is the production of more virions, released by exocytosis, and the death of the infected host cell due to the metabolic burden of this intensive virion production.
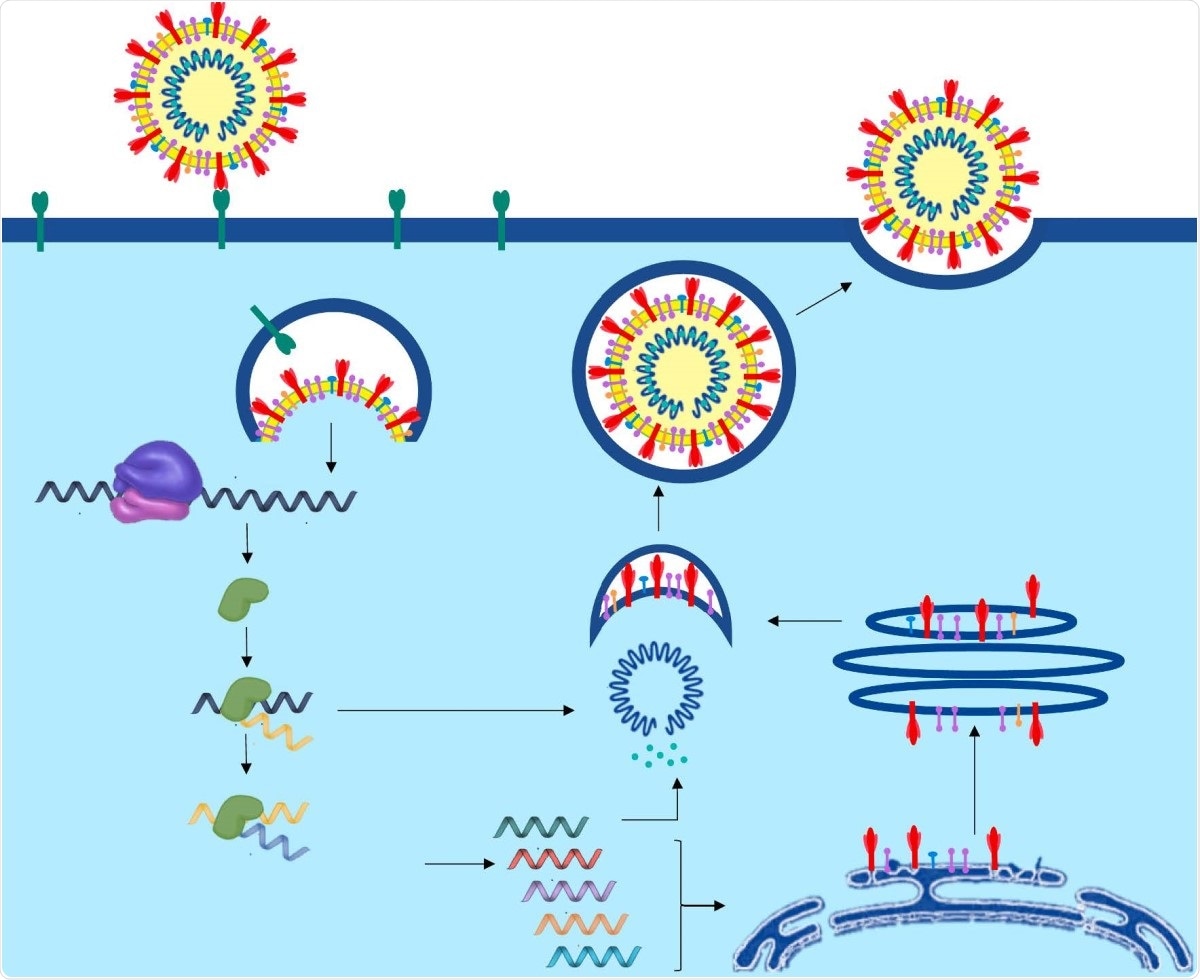
The life cycle of SARS-CoV-2 from entry and viral replication to viral release in the human cell.
While most infections are asymptomatic or mild, in a small but significant minority, they proceed to viral pneumonia, acute respiratory distress syndrome (ARDS) and death. In this phase, there is a remarkable flood of inflammatory cytokines like I2, IL17, TNFα and GCSF. Mortality is high among elderly and already sick individuals, at 50-75% versus the 2-3% mortality generally accepted as the overall case fatality rate.
The immune system of the host responds by innate and adaptive responses to the presence of the virus. Viral recognition is by toll-like receptors (TLR) 3,7 and 8, which are pathogen pattern recognition receptors. This leads to enhanced interferon (IFN) release.
The B cells initiate the humoral response by forming anti-N antibodies early in the infection, with anti-S antibodies being formed 4-8 days after symptom onset. IgM, IgA and IgG antibodies have been identified, the first two within about 5 days from the earliest symptoms, and IgG after about 2 weeks. IgG persists for several months once produced. Severe infection has been observed to correlate with a more robust antibody response. Again, viral clearance from the upper respiratory tract is more likely with a weak antibody response compared to a strong one.
Neutralizing antibodies prevent the viral entry into the host cell, but non-neutralizing antibodies may facilitate the action of antigen-presenting cells and other immune cells. However, antibodies to other coronaviruses, including seasonal coronaviruses, may promote viral attachment to immune cells via their engagement with the Fc receptor on these cells' membranes. This does not lead to replicative infection, being non-ACE2 mediated.
However, it does cause virus-induced myeloid cell stimulation and aggravated tissue damage, along with a reduction in T cells and T cell dysfunction. In acute infection, the lymphocyte count is undetectable or very low. Early cell-mediated immune response may lead to effective viral clearance in mild infection, with the innate immune response inhibiting cytokine release. Severe COVID-19 is associated with a cytokine storm and a poor outcome.
Interferons in immunity
Interferons are natural proteins with a broad spectrum of action against viruses and inflammatory processes. They bind to specific cell surface receptors to activate the JAK-STAT pathway, which in turn mediates the transcription of IFN-stimulated genes (ISGs). There are three types, I, II and III. The first is generated by infected and immune cells, while type II, represented by the single member IFN-γ, is also secreted by immune cells. Type III is secreted by epithelial cells, among others.
Molecular model of interferon-alpha IFN-alpha, 3D illustration. Image Credit: Kateryna Kon / Shutterstock
Type I and II are pro-inflammatory, facilitating the destruction of infected cells, deactivating viruses by antibodies, cleaning up cellular and viral debris.
Type III are anti-inflammatory, inhibiting viral replication within the infected cells and promoting epithelial barrier integrity.
Type I IFNs are thus essential for early viral containment within the infected cells, but are related to a fatal course in the current infection, due to a dysregulated immune response. This is signaled by rises in the level of 38 cytokines at least, 15 of which relate to lung damage.
SARS-CoV-2 produces less type I IFN activation compared to earlier pathogenic coronaviruses, but all viruses in this family share the ability to evade the immune response and produce type I IFN.
According to a Chinese study, early use of IFN-α2b can reduce in-hospital mortality when given early, but in the late stages, it increases mortality in COVID-19.
IFN-β-1a could also be useful in viral diseases like hepatitis and may reduce mortality and cut short the period of illness in COVID-19. Trials on its inhalable form, SNG001, are going on in patients with chronic obstructive pulmonary disease (COPD) or asthma, with very encouraging results. IFN-β-1b might also be useful in early disease onset, since it promotes endothelial barrier integrity and increases adenosine levels, but firm data is lacking.
IFN-γ is a stimulant of cytokine expression via immune cells' activation, including monocytes and T cells. It expands cytotoxic T lymphocyte clones by introducing major histocompatibility complex (MHC) class I, or granzyme B, and MHC II receptors. It is key to limiting the replication and distribution of the virus and could be useful as an inexpensive therapeutic for dangerously ill patients, pending the availability of more effective and specific drugs.
IFN-λ is a type III IFN acting on epithelial cells, and induces antiviral activity within epithelial cells. Pegylated IFN-λ1 (peg-IFN-λ1) is available at present. While it does not promote a cytokine storm, its effect on the immune response must be studied further, as well as any effect on epithelial and other cell proliferation.
Interferons in COVID-19 therapy
At present, the treatment of COVID-19 depends on supportive measures, but some antivirals have been repurposed for use against SARS-CoV-2, such as remdesivir, corticosteroids, along with monoclonal antibodies and stem cell therapies.
Some researchers have suggested that the early implementation of IFNs could reduce viral counts and clinical symptoms, though it is unlikely to reduce the fatality rate. IFN-α has been shown to relieve symptoms and shorten the clinical course, in COVID-19, as in earlier studies on SARS, pneumonia, hand, foot and mouth disease (HFMD) in children and bronchiolitis.
Further research will be required to define the role and treatment protocols using various IFNs, their cellular pathways and mechanisms of action, and the specific cellular targets of IFN therapy. Also, the contribution of other drugs such as antivirals, antibiotics and corticosteroids will be a fruitful area of research. Clinical trials underway in China to assess the potential of such therapies, and the results should be available next year. The authors sum up: "Combination therapy with various drugs in different stages of this pandemic could be more efficient than monotherapy."