There has been immense concern as to whether protective immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) lasts long enough to prevent reinfection soon after recovery.
A new study published by a team of scientists in Spain indicates that neutralizing immunity can last for up to 8 months, despite a steep drop in antibody titer over this time. Their findings were released on the bioRxiv* preprint server in November 2020.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The study included 210 patients with COVID-19 infection confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) testing for viral RNA. The cases dated from the first and second waves of the pandemic in Catalonia, Spain. The group comprised 106 with mild infection and the rest with serious infection requiring hospitalization due to respiratory distress.
The samples were obtained over around 240 days of follow-up. Most of the participants were found to develop antibodies that neutralized pseudoviruses expressing SARS-CoV-2 antigens. However, the maximal titer in mild cases was 10 times lower at peak level relative to that in hospitalized patients.
During the acute phase, the number of observations taken on hospitalized individuals was higher, allowing the shape of the early response to be outlined in both waves of the pandemic. The researchers found that within 30 days of the first symptom, there was a rapid increase in neutralizing activity, from a half-maximal neutralization activity at day 10 to 80% (3.97 logs) maximal by day 14.
The slope of neutralizing activity thereafter was almost flat, with the half-life being about 2,100 days. On the other hand, in hospitalized patients, the neutralizing activity declined in two phases, first a rapid fall with a half-life of 31 days until day 80, with a slower decline thereafter, with a half-life of about 750 days.
Since the exact threshold at which new infection is prevented is unknown, the researchers decided to evaluate neutralizing activity at the end of their study period. They estimated a value of 2.72 and 3.16 log neutralizing activity for mild and hospitalized patients, respectively. This value was stable and agreed with the measured values between days 135 and 242, the midpoint being day 180.
The distribution of values within this period also showed the median among mild cases to be 2.5, and 3 for hospitalized patients.
High protection among hospitalized patients
It is estimated that a neutralizing activity between 1:161 and 1:3,082 is potent enough to prevent new infection, and if so, reinfection would probably not happen above 1:1,250. Interestingly, of the 23 hospitalized individuals whose serum neutralizing activity was measured beyond day 135, 21 (90%) fulfilled this criterion. In other words, 90% of these patients could be considered to have long-term neutralizing activity.
Of the participants in the other group, only 42% had long-term neutralizing activity. While this classification is based on an arbitrary cut-off, it suggests that durable neutralizing activity is more likely after severe infection, despite the sharp initial drop.
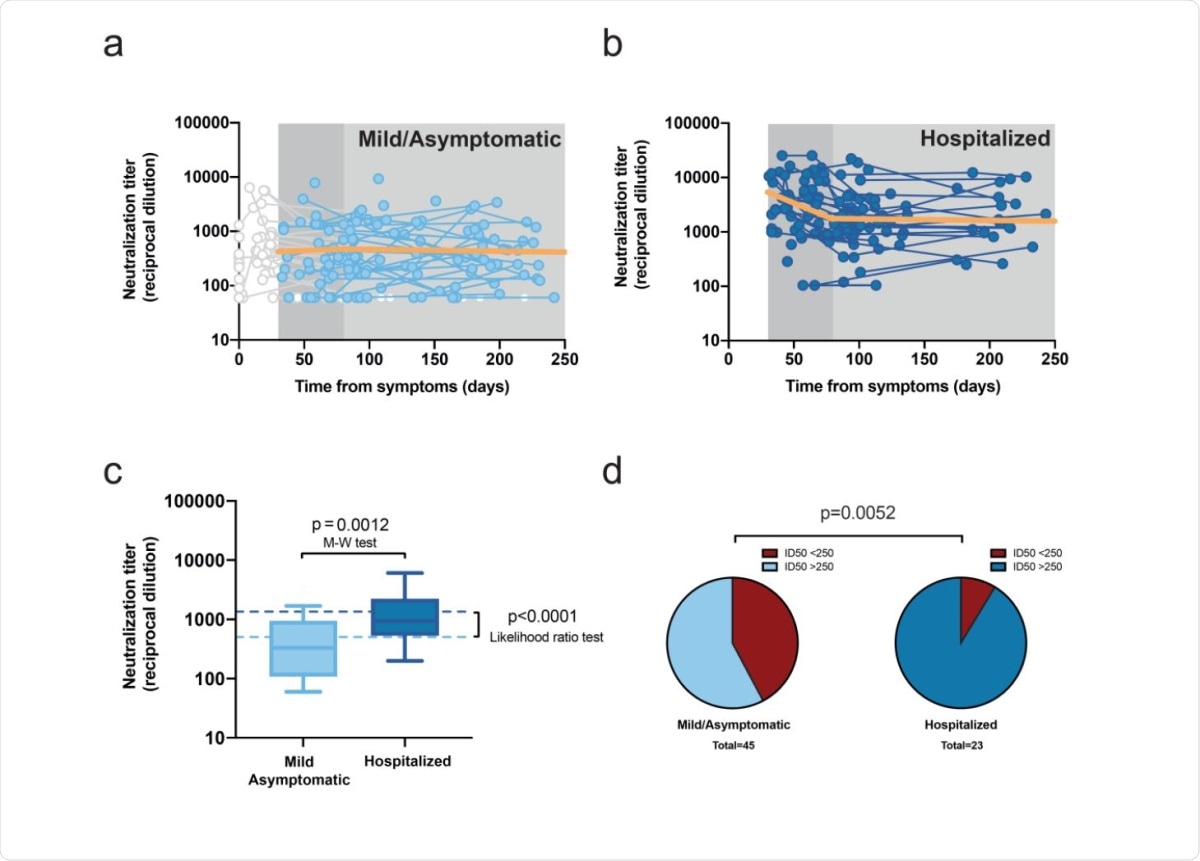
Longitudinal analysis of neutralizing activity. a, Individual measurements (dots) and linear mixed model (solid orange line) of the longitudinal analysis for mild or asymptomatic individuals beyond day 30 (single-phase slope -0.00014; p=0.75, likelihood ratio test; estimated half-life 2,134 days). Time points preceding day 30 as well as participants only showing undetectable titers were excluded from the analysis, values are shown but grayed out. b, the corresponding analysis for hospitalized individuals (the slopes of the linear fit for the first and second phase were -0.0096 [p=0.0002] [half-life 31 days] and -00004 [half-life 753 days] [p=0.78], respectively). c, Distribution of neutralizing activity six months after infection in both disease severity groups. Experimental values of mean neutralizing activities in the period 135-to-242 days as summarized in box-plots (as in Figure 1a; Mann-Whitney test for comparative analysis) and modeled data as dotted lines (likelihood ratio test for comparative analysis). d, Frequency of long-term neutralizers (i.e., individuals with mean neutralizing activity >250 in the 135-242 days period) in each severity subgroup (Chi square test p value is shown).
Do neutralizing activity patterns follow the antibody titer?
To answer this, the researchers looked at the serum IgG titers in 28 participants, 14 mild/asymptomatic, and 14 hospitalized participants, who had the longest follow-up. They found that the fastest decay was seen with IgG anti-nucleoprotein (NP) antibodies, but anti-spike (S) S2 subunit and receptor-binding domain (RBD) IgG antibodies also followed a steady linear decay pattern. The half-life for these antibodies, after day 30, was 59, 108 and 86, respectively. These values agree with earlier research carried out over a period of 160 days.
From this limited dataset, It appears that anti-S2 titers were markedly increased in hospitalized patients at the end of the study period. This group again showed the two-phase model of decay.
The researchers explain that this phenomenon is due to an increasing number of IgG mutations, which shift antibody production towards those with greater neutralizing power. These are better at inhibiting virus entry and infection. This explains how a lower specific antibody titer over time can still be accompanied by greater stable neutralizing activity.
Again, the current study included infected individuals from both waves of the pandemic. This allows scope for the possibility that many who were infected in the first wave were still exposed to a high viral presence around them, which could cause a higher humoral response.
The researchers attribute the contrast between their finding of a two-phase pattern of neutralizing activity, and the rapid fall in antibodies observed in earlier research, to the inclusion of many individuals who were in the early phase of acute infection in the latter group of studies. Thus, it may be hoped that natural infection or vaccine-mediated herd immunity will induce long-term neutralizing activity.
Implications and future directions
The findings offer hope that more severe COVID-19 infection is associated with long-term protection against reinfection. However, data from earlier human coronavirus studies show that antibody titers do fall within 1-2 years from infection, and no vaccine data is available for these viruses to provide any comparisons. Thus, continued follow-up of this group of individuals will help understand how the immune response changes over the long term.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Pradenas, E. et al. (2020). Stable neutralizing antibody levels six months after mild and severe COVID-19 episode. bioRxiv preprint. doi: https://doi.org/10.1101/2020.11.22.389056,https://www.biorxiv.org/content/10.1101/2020.11.22.389056v2
- Peer reviewed and published scientific report.
Pradenas, Edwards, Benjamin Trinité, Víctor Urrea, Silvia Marfil, Carlos Ávila-Nieto, María Luisa Rodríguez de la Concepción, Ferran Tarrés-Freixas, et al. 2021. “Stable Neutralizing Antibody Levels 6 Months after Mild and Severe COVID-19 Episodes.” Med 2 (3): 313-320.e4. https://doi.org/10.1016/j.medj.2021.01.005. https://www.cell.com/med/fulltext/S2666-6340(21)00035-0.