The relentless spread of COVID-19 implies that the reopening of our society and the return to our previous lifestyle depends on the development and implementation of safe and effective treatment modalities or vaccines.
This highlights the importance of appraising determinants of protective immunity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a causative agent of COVID-19. A crucial question mark at the moment is what immune responses can be linked to protective immunity.
Consequently, there is a dire need for standardized, reproducible antibody assays to establish correlates of risk and protection. As the scientific progress continues at an unprecedented pace, a myriad of neutralization assays utilized by different laboratories and companies have emerged.
In the current study, US researchers (led by Dr. Anton M. Sholukh from the Vaccine and Infectious Diseases Division at the Fred Hutch Cancer Research Center in Seattle) used plasma samples from convalescent individuals with mild/moderate COVID-19 in order compare three different SARS-CoV-2 neutralization platforms.
Assays and antibodies
In this study, the researchers compared live recombinant SARS-CoV-2 assay, a surrogate ELISA-based test, and pseudovirus-based assays. Furthermore, two different pseudovirus packaging techniques (with the use of lentivirus and vesicular stomatitis virus) and three different cell lines were identified and further characterized.
The researchers also explored the correlation between neutralization and the concentration of Immunoglobulin G (IgG) antibodies against SARS-CoV-2 nucleoprotein, spike glycoprotein, and the receptor-binding domain. All sera samples were derived from a localized outbreak in a single region of the western US.
"With the set of cell lines used for the assays in this study, we were able to address questions regarding the influence of proteolytic cleavage of SARS-CoV-2 spike on virus neutralization by serum antibodies, which is important for choosing an assay that would provide more physiologically relevant outcomes", explain study authors in this preprint paper.
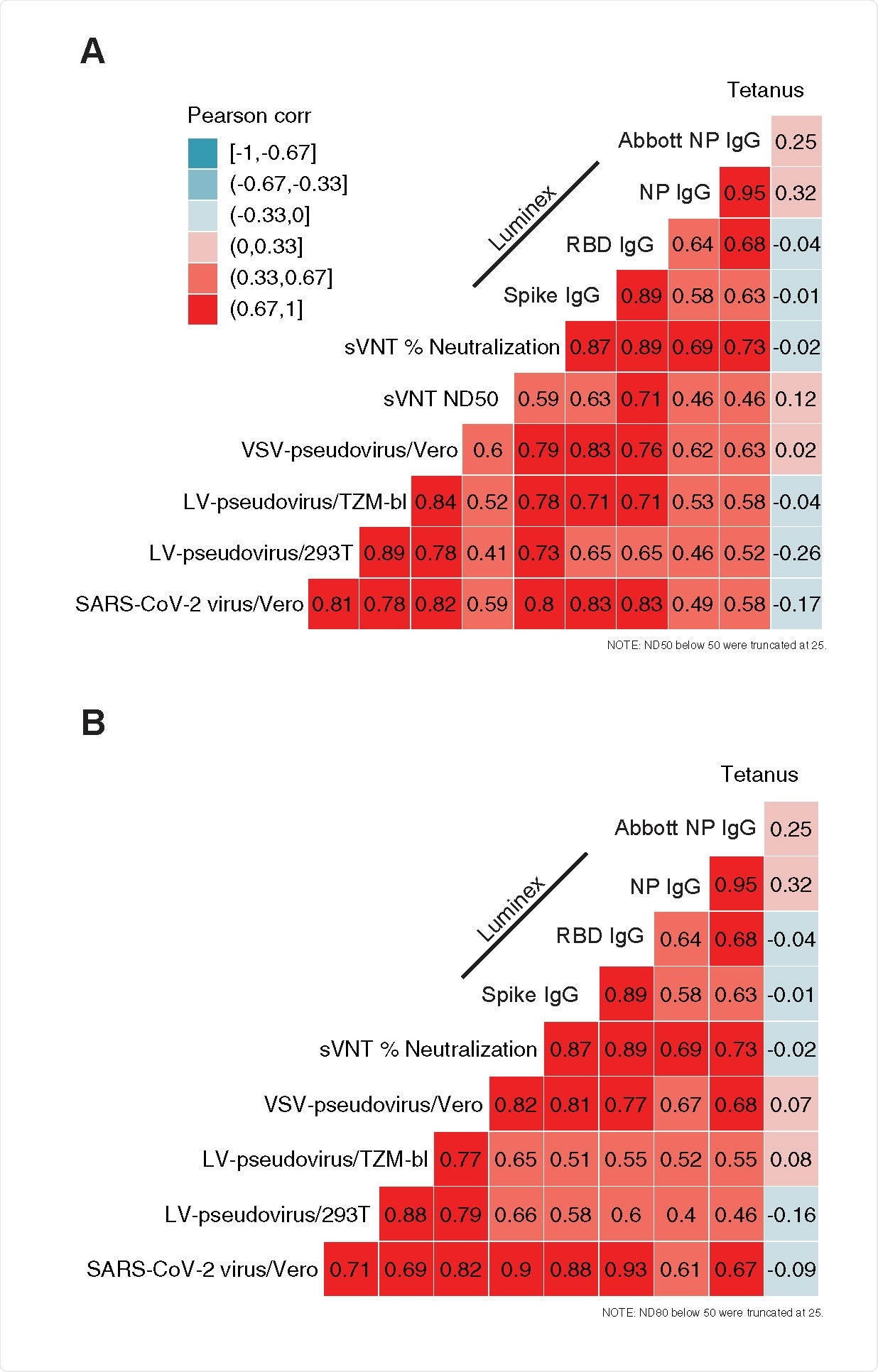
Correlation among assay readouts measuring neutralization or antigen-specific IgG concentration in plasma. Heatmap color is determined by the Pearson’s correlation coefficient (r, annotations). Each panel includes either ND50 titers (A) or ND80 titers (B) and their correlation with sVNT % neutralization, SARS-CoV-2 specific IgG concentration (Luminex bead-based assay), the quantitative index of the Abbott nucleoprotein assay and tetanus toxoid-specific IgG concentration.
Comparable neutralization readouts
Readouts of the 50% and 80% neutralization titers in cell-based assays showed a high correlation with each other, as well as with the concentration of IgG antibodies against SARS-CoV-2 receptor binding domain and spike glycoprotein.
Moreover, the results of the ELISA-based surrogate virus neutralization test were also positively correlated with the other neutralization assays used in this comparison; nonetheless, the correlation was modest in this case.
The observed correlation between nucleoprotein-specific IgG and neutralization was lower when compared to the correlations of spike and receptor binding domain-specific IgG with neutralization. This is compatible with described mechanisms of neutralization, which entail binding and, potentially, blocking the spike: ACE2 receptor binding domain.
Even though no significant difference has been observed in virus titer 48 hours after infection on the wild-type Vero cells and Vero cells expressing the enzyme furin, during the early time points cells expressing protease demonstrated higher viral titers.
Implications for use
"In conclusion, the surrogate assay can be used cautiously as an alternative to cell-based assays to obtain preliminary qualitative results, to rapidly distinguish between samples with high and low neutralizing potency, and when a cell-based assay is not available or reasonably feasible", conclude study authors.
Such surrogate assay can be performed with the use of biotinylated soluble ACE2 receptor competing with serum antibodies for a binding spot on immobilized spike protein or receptor-binding domain, or vice versa, when soluble receptor binding domain is used and immobilized ACE2 receptor.
Although this research endeavor demonstrates high concordance between cell-based assays with live and pseudotyped virions, further studies are needed as countries worldwide gear up for expeditious roll-outs of COVID-19 vaccines.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.