Nearly a year into the coronavirus disease 2019 (COVID-19) pandemic, targeted vaccinations against the causative pathogen – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – have commenced in the United States and the United Kingdom
Before the approval of a vaccine by regulatory bodies, human trials are needed to prove efficacy and safety.
A new multicenter study has shown that blinded crossover and continued follow-up of trial participants can help assess vaccine durability and potential delayed enhancement of disease.
Candidate vaccines to prevent COVID-19 are now conducting large-scale phase 2 placebo-controlled randomized clinical trials. Some of these vaccines showed substantial short-term efficacy.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Vaccine approval
Early in the coronavirus pandemic, many scientists and pharmaceutical companies developed vaccines in the hopes of putting an end to the ongoing global health crisis.
According to the World Health Organization (WHO), there are more than 200 candidate vaccines against SARS-CoV-2. Of these, about ten vaccines are in the last phase of clinical trials, which involve human testing.
Early results from at least three trials suggest high efficacy that exceeds the FDA guidance threshold of 50 percent for symptomatic disease and severe disease. Other concerns regarding vaccine trials include their potential effects on subgroups, such as the elderly and obese individuals. These individuals have weakened responses to vaccines.
COVID-19 phase 3 vaccine trials
Current COVID-19 phase 3 vaccine trials are large and designed to obtain at least 150 cases of clinical disease in a short period.
During the phase 3 trial, the best way to evaluate the long-term safety and durability is by continued blinded follow-up of the original arms, and at some point, placebo volunteers should be offered the vaccine.
However, the timing of offering vaccines to placebo volunteers is complex, as individual risk is assessed in terms of the availability of the vaccine outside the trial, societal perceptions of fairness, and the social value of added information.
Perceiving equity among participants will influence the retention and will determine how long blinded placebo control continues. Meanwhile, following cross-over, the randomized trial remains, but compared to the original vaccine arm and the original placebo group vaccination.
This way, scientists can assess the waning vaccine efficacy or associated enhanced disease. To ensure that the trial has the highest quality, blinded follow-up crossover is needed, wherein placebo recipients get vaccinated, while the vaccine recipients receive a placebo.
The study
The study, which appeared on the pre-print medRxiv* server, shows that apart from the durability, continued follow-ups provide a means to measure the post-vaccination immune response in the newly vaccinated placebo recipients is essential. It also allows a pivot to a randomized trial of a booster dose of the vaccine.
Following vaccination of the placebo group, the researchers showed that placebo-controlled vaccine efficacy could be obtained by assuming that the benefit of vaccination over time has the same profile for the original vaccine recipients and the placebo crossovers. Hence, the method enables the estimation of vaccine durability and possible vaccine-linked enhanced disease.
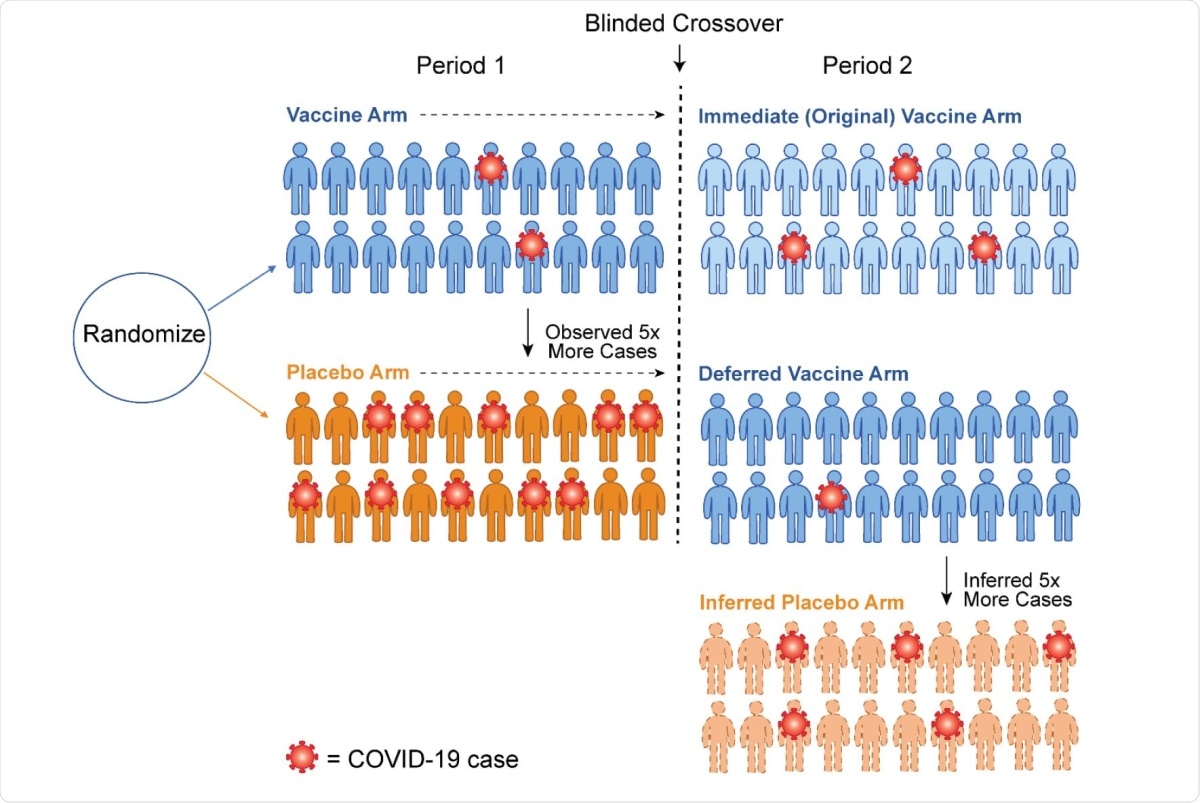
Schematic of how crossover allows imputation of the case counts for an inferred placebo group. Following crossover, we assume the vaccine efficacy in Period 2 for the newly vaccinated (Deferred Vaccine Arm) is the same 80% that was observed in the newly vaccinated (Immediate Vaccine Arm) in Period 1. This logic implies that a counterfactual placebo group of 20 volunteers would have about 5 cases. Thus the vaccine efficacy for the original vaccine arm in Period 2 has waned to 100% (1 – 3/5) = 60%.
What the study found
The researchers found post-crossover helps estimate the effectiveness of the vaccine. It can help shed light on the vaccine durability, determine waning efficacy, and identify late enhancement of the disease. However, these results are less reliable than those obtained by a standard trial, wherein the placebo group is maintained.
Further, the team noted that vaccine efficacy for post-crossover periods relies on previous vaccine effectiveness estimates. Thus, longer pre-crossover periods with a higher number of cases give better estimates of late vaccine efficacy.
This means that an open-label crossover may cause riskier behaviors during the immediate crossover period, which can influence vaccine efficacy estimates.
With an unblinded crossover, there is a real concern that immediately after crossover the newly unblinded original vaccine recipients will forgo masks and social distancing while the newly unblinded placebo volunteers will not,” the researchers explained.
Nevertheless, if some of the volunteers opt to become unblinded, continued follow-up of these participants should be done to maximize information. The trial heads can ask simple questions to assess planned and actual risk behavior, along with comorbidities, to help adjust for biases caused by unblinding.
Continued blinded follow-up in the original arms is optimal to assess vaccine efficacy over time and is endorsed by the FDA in their guidance about COVID-19 vaccine development,” the researchers concluded in the study.
They added that continuing with the follow-up allows for a doubling of volunteers. They can contribute to an immune correlates analysis, allowing for a quick pivot of a trial of boosters, in case the vaccine shows fading efficacy.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references:
- Preliminary scientific report.
Follman, D., Fintzi, J., Fay, M., Janes, H., Baden, L. Sahly, H. et al. (2020) Assessing Durability of Vaccine Effect Following Blinded Crossover in COVID-19 Vaccine Efficacy Trials. medRxiv. doi: https://doi.org/10.1101/2020.12.14.20248137, https://www.medrxiv.org/content/10.1101/2020.12.14.20248137v1
- Peer reviewed and published scientific report.
Follmann, Dean, Jonathan Fintzi, Michael P. Fay, Holly E. Janes, Lindsey R. Baden, Hana M. El Sahly, Thomas R. Fleming, et al. 2021. “A Deferred-Vaccination Design to Assess Durability of COVID-19 Vaccine Effect after the Placebo Group Is Vaccinated.” Annals of Internal Medicine 174 (8): 1118–25. https://doi.org/10.7326/m20-8149 https://www.acpjournals.org/doi/10.7326/M20-8149.