Accurate coronavirus disease 2019 (COVID-19) diagnosis can interrupt transmission, aid clinical management, and help proper allocation of resources to isolation, contact tracing, and therapy. In many low- and middle-income settings with huge outbreaks, COVID-19 diagnosis efforts using highly sensitive molecular tests have exceeded laboratory capacity.
There is a pressing need to understand the conditions under which the use of Ag-RDTs for COVID-19 diagnosis would be preferable to other methods such as NAAT and/or clinician judgment alone.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Comparing the effectiveness of Ag-RDT, NAAT, and clinical judgment alone in COVID-19 diagnosis
A team of researchers from the Johns Hopkins University, USA; Imperial College London, UK; and Foundation for Innovative New Diagnostics, Switzerland, recently compared the effectiveness of Ag-RDT, NAAT, and clinical judgment alone in the diagnosis of COVID-19 in symptomatic patients. They studied 2 scenarios:
- A hospital setting with high prevalence with a 24-hour NAAT turnaround
- An outpatient setting with lower prevalence with a 3-day NAAT turnaround.
Their study has been published on the preprint server, medRxiv*.
The researchers simulated transmission from cases and contacts and relationships between time, transmission, viral burden, and detection of cases. They used decision curve analysis to compare different diagnostic approaches, and estimated the time- and infectivity-dependent benefit of each positive diagnosis.
Results show greater net benefit with NAAT in the hospital setting and with Ag-RDT in the outpatient setting
In the primary analysis that compared Ag-RDT and NAAT, they found that greater net benefit was achieved with NAAT in the hospital setting and with Ag-RDT in the outpatient setting. In the hospital setting, Ag-RDT became more beneficial when NAAT turnaround times surpassed 2 days or when relative Ag-RDT sensitivity increases to at least 95% during acute illness.
“Ag-RDTs can also offer greater benefit than NAAT-based testing in hospitalized populations when NAAT delays are long or Ag-RDT sensitivity surpasses 95% that of NAAT.”
Similarly, in the outpatient setting, NAAT was found to be more beneficial when NAAT turnaround time remained under 2 days or when patients properly isolated while waiting for test results. Clinical judgment was preferred only if clinical diagnoses generated a strong clinical and public health response and false-positive diagnoses caused minimal harm.
“Although NAAT offers higher sensitivity … than Ag-RDT, the fact that Ag-RDT delivers results more rapidly and identifies the most highly infectious individuals can make it equivalent or superior to NAAT in averting transmission.”
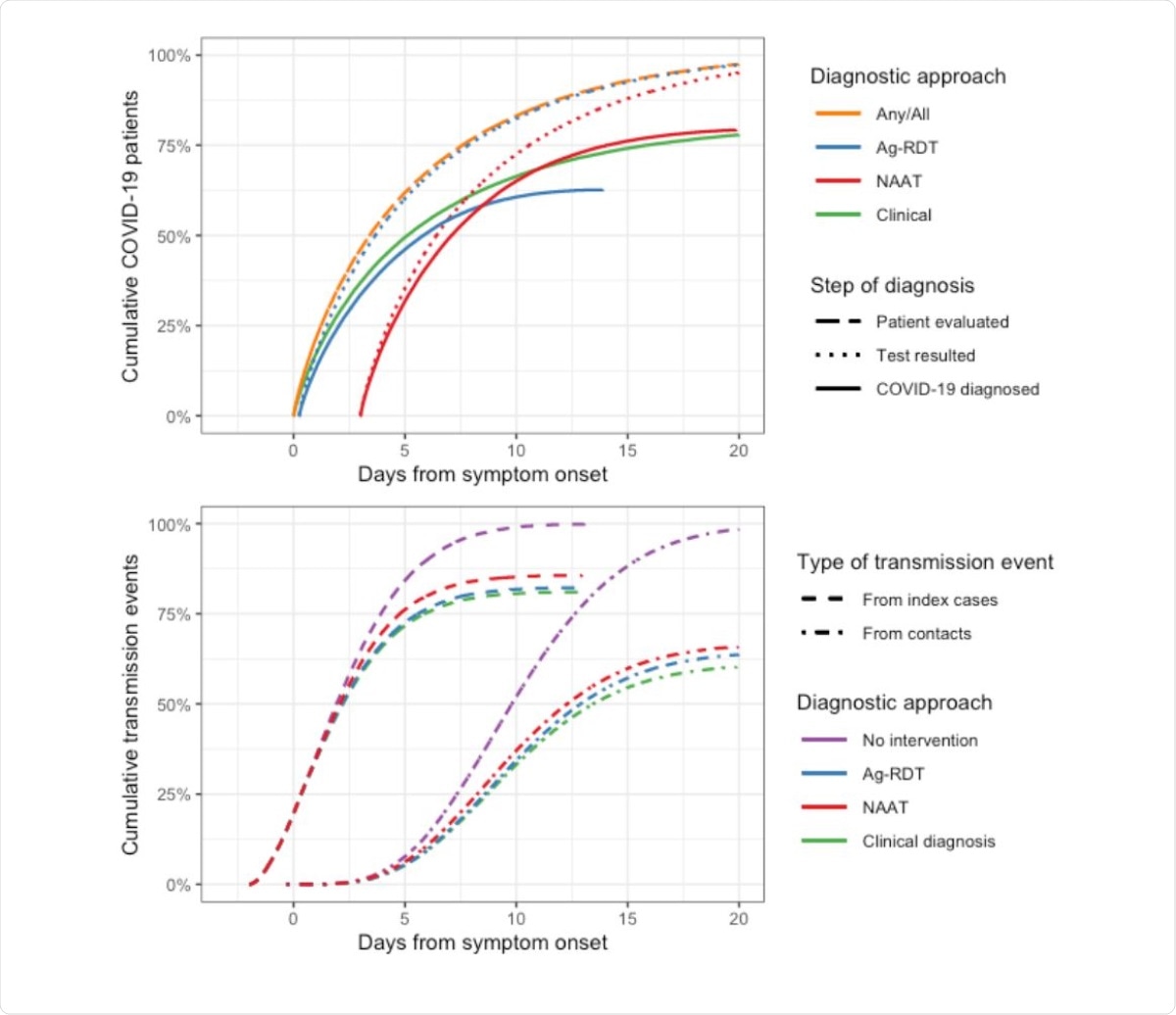
Timing of testing, test results, and transmission events prevented by each assay, in the outpatient setting. All times are shown relative to symptom onset. The top panel shows when patients with COVID-19 are evaluated (i.e. when they present to care and any diagnostic specimens are collected) and when diagnostic results are received (with “Test resulted” showing all results and “COVID-19 diagnosed” showing the cumulative proportion of patients with a positive result). While 50% of patients are evaluated within 3.5 days of symptom onset, some patients present after more than two weeks of symptoms (orange dashed line). These delays reduce the overall proportion of cases detected by NAAT and Ag-RDT from 90% and 77% (their absolute sensitivities in early illness) to 80% and 62%, respectively (solid lines). Although NAAT ultimately detects a greater proportion of cases than Ag-RDT, those detected by Ag-RDT are detected sooner compared to NAAT, are more infectious on average, and are in a more acute stage of illness, allowing Ag-RDT to have a greater effect on transmission than NAAT (bottom panel).
Ag-RDT may offer more net benefit than either NAAT or clinical judgment with >2 day NAAT turnaround times
As Ag-RDTs for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) diagnosis become more widely available, it is crucial to identify the settings under which they offer greater benefit compared to NAAT or clinical judgment alone. The researchers used a novel adaptation of net benefit analysis and demonstrated that Ag-RDT could outperform NAAT under outpatient conditions that include multiple-day turnaround times for NAAT, even if Ag-RDT has comparatively lower sensitivity.
“Our novel application of decision curve analysis to COVID-19 demonstrates the importance of accounting for factors beyond sensitivity and specificity when using this tool to evaluate infectious disease diagnostics.”
They also demonstrated when Ag-RDT would be preferable over NAAT in the hospital setting, including NAAT turnaround times of over 48 hours or Ag-RDT assays that have a relative sensitivity >95% compared to NAAT during acute illness.
Based on these findings, the authors concluded that Ag-RDT may provide more net benefit than either NAAT or clinical judgment in diagnosing symptomatic COVID-19, when NAAT turnaround times are over 2 days. NAAT is likely to be optimal for patients who are hospitalized with prolonged symptoms before admission.
“In conclusion, our modified decision curve analysis demonstrates that for symptomatic individuals, a moderately sensitive Ag-RDT could offer greater net benefit than either NAAT testing or clinician-driven diagnosis for COVID-19.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Emily A Kendall, Nimalan Arinaminpathy, Jilian A Sacks, Yukari C Manabe, Sabine Dittrich, Samuel G Schumacher, David W Dowdy. (2020) Understanding the net benefit of antigen-based rapid diagnostic tests for COVID-19: An enhanced decision-curve analysis. medRxiv preprint server. doi: https://doi.org/10.1101/2020.12.16.20248357,
https://www.medrxiv.org/content/10.1101/2020.12.16.20248357v
- Peer reviewed and published scientific report.
Kendall, Emily A., Nimalan Arinaminpathy, Jilian A. Sacks, Yukari C. Manabe, Sabine Dittrich, Samuel G. Schumacher, and David W. Dowdy. 2021. “Antigen-Based Rapid Diagnostic Testing or Alternatives for Diagnosis of Symptomatic COVID-19.” Epidemiology 32 (6): 811–19. https://doi.org/10.1097/ede.0000000000001400. https://journals.lww.com/epidem/Abstract/2021/11000/Antigen_based_Rapid_Diagnostic_Testing_or.7.aspx.