The emergence of the coronavirus disease 2019 (COVID-19) pandemic has literally changed the way we lead our lives. The rapid spread of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) demands accurate and mass-scale diagnosis to prevent the transmission of this virus.
But there is a scarcity of rapid diagnostic tests along with inaccessibility of advanced instrumental techniques to all the diagnostic centers, especially the remote ones. This major shortcoming contributes to the larger spread of the virus followed by an increased mortality rate.
The major motivation of our work was to develop a translatable solution for this problem that can identify COVID-19 positive cases possibly within a few minutes without access to any of the advanced instrumental techniques.
Please describe the sensor you have developed. How did you develop this sensor and how does it detect COVID-19?
This is a molecular test. We discovered new molecules that can bind to the viral genetic material with high specificity. We apply these molecules in combination with nanoparticles made from gold. These materials are deposited on a paper-based substrate containing highly conductive graphene.
Because of the high conductivity of both gold and graphene, this platform becomes ultrasensitive at detecting changes in electrical signals. As the viral genetic material undergoes hybridization with the molecular probes, it causes a change in the sensor’s electrical response.
This process accelerates the electron transfer and when spread over the sensing platform, results in an increase in the output signal and indicates the presence of the virus.
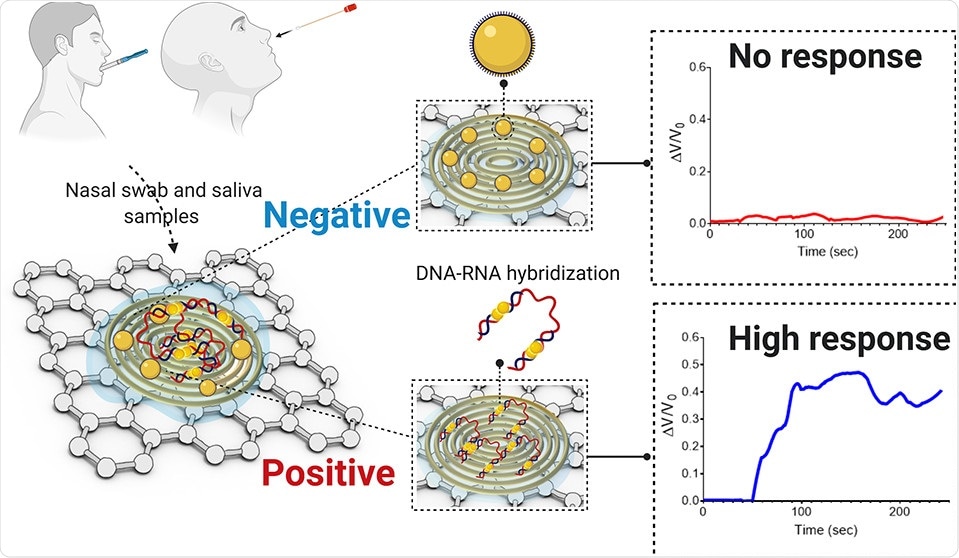
Abstract. Image Credit: ACS Nano 2020
How have you tested the sensor?
The sensor passed extensive validation in the laboratory setting using COVID-19 viral RNA. Once that was achieved, the clinical validation was performed with human nasopharyngeal swab samples collected from COVID-19 positive patients.
What current methods of COVID-19 testing are available?
At present, COVID-19 is being diagnosed by four of the major techniques: (1) reverse-transcription polymerase chain reaction (RT-PCR) and gene sequencing; (2) an antibody-based serological/ immunological assay and (3) chest computed tomography (CT) and (4) antigen-based techniques.
However, the inadequate access to advanced instrumental techniques, often cannot report positive COVID-19 cases at its initial presentation leading to the spread of this infectious disease to a wider community. Several reports indicated that antigen-based tests can lead to erroneous results.

RT-PCR COVID-19 Test. Image Credit: anyaivanova/Shutterstock.com
Why is this sensor so advantageous and how could it change the course of the global pandemic?
Getting a molecular test done at a point of care is not a trivial task. Our results indicated that we can achieve remarkable sensitivity, specificity, and limits of detection. As a matter of fact, we can detect ~6 viral copies/microliter in samples collected from COVID-19 positive patients.
Moreover, the advantage of this technology is that we do not need an additional amplification step to reach such remarkable sensitivity. This is critical because the amplification step is often performed at high temperatures. The requirement of high temperature makes it difficult in practice to translate any molecular diagnostic tests that involves a heating step.
Our technology is devoid of an amplification heating step and generates results in minutes. The remarkable simplicity and rapid sample to response time make this technology advantageous.
Could your research be applied to other diseases?
Yes. We have developed a platform technology that can be applied to detect many other diseases by swapping the chemical motif that is responsible for binding to the target genetic material.
We have demonstrated that our invention can successfully be applied to other infectious diseases from both bacterial and viral origins.
When could this sensor become widely accessible, and what are the next steps for your research?
My team which included multidisciplinary expertise of Dr. Parikshit Moitra (a biological chemist and a junior faculty in my lab), Ms. Maha Alafeef (a third-year Graduate Student in Bioengineering), and Mr. Ketan Dighe (an electrical engineer and a Research Fellow) has been working relentlessly to develop a prototype.
We have successfully licensed our plasmonic platform to a company. Currently, that test is being developed for commercialization. We are anticipating that the plasmonic technology can be brought into the market very quickly.
The company is also seeking approval for emergency use authorization (EUA) from the FDA. For the electrochemical approach, we are having early discussions with multiple interested companies.
.jpg)
Image Credit: Corona Borealis Studio/Shutterstock.com
Where can readers find more information?
About Professor Dipanjan Pan
Prof. Dipanjan Pan, MS, Ph.D., is a renowned expert in nanomedicine, molecular imaging, drug delivery, and biosensing. He is presently a tenured Full Professor in Diagnostic Radiology & Nuclear Medicine, Pediatrics and Chemical, Biochemical and Environmental Engineering at the University of Maryland Baltimore School of Medicine and University of Maryland Baltimore County.
Before moving to Maryland, he was a tenured Associate Professor and Associate Head of the department in Bioengineering and Materials Science and Engineering and Institute of Sustainability in Energy and Environment at University of Illinois, Urbana-Champaign. He administratively directs the Nanofabrication and Characterization Core at CBOTH and a Director for the Investigational Probe Design Resources in Radiology.
Prof Pan’s lab uniquely merges fundamental chemistry, biology, and engineering to bring a solution to today’s healthcare problems. Over the years, this research has resulted in more than 200 high impact peer-reviewed publications in scientific journals, numerous conference abstracts and has been supported by multimillion-dollar external funding from NIH, NSF, DoD, American Heart Association, and other private/foundational funding sources.
Prof. Pan edited and co-written two books published by Taylor and Francois (Nanomedicine: A Soft Matter Perspective, ISBN-13: 978-1466572829) and Springer (Personalized Medicine with a Nanochemistry Twist: Nanomedicine (Topics in Medicinal Chemistry, ISBN-13: 978-3319335445). He holds multiple patents (>30 US granted patents and applications), numerous invention disclosures several ongoing clinical trials. He is the founder of three University-based early start-ups. He is the CEO/President for a biotechnology start-up Vitruvian Bio, dedicated to developing novel image-guided therapies. He co-founded InnSight Technologies dedicated to developing next-generation biosensing solutions for ocular diseases.
His other company KaloCyte, Inc, which he co-founded with his clinical collaborators, develops an artificial oxygen career. He acts as a Chief Technology Officer for all the spin-offs from his lab. His technology has been licensed for commercial development multiple times. He serves as a study section review board member for NIH, CDMRP (DoD), NSF, and a multiple review committee member for the American Heart Association.
In 2016 he received Nanomaterials Letter (NML) Researcher award, in 2017 a Young Innovator Award from Biomedical Engineering Society (BMES), and a Deans Award for Research Excellence in 2018. He is an elected fellow of the Royal Society of Chemistry, a Fellow of the American Heart Association, and an elected fellow of the American College of Cardiology.
Professor Pan is an Associate Editor for WIRES Nanomedicine and Nanobiotechnology, and an editorial advisory board member of Molecular Pharmaceutics (ACS).