As the coronavirus disease 2019 (COVID-19) pandemic continues to claim thousands of lives globally every day, new vaccines are being rolled out, with reported preventive efficacies of 94%, for the Moderna vaccine, 95% for the Pfizer, BioNTech vaccine, and an overall efficacy of 70% for the Oxford-AstraZeneca vaccine. This has awakened hopes that normal everday life may soon be possible again. A recent study published on the preprint server medRxiv* in December 2020 reflects on the possibility of using a single-dose vaccine regimen to accomplish large-scale vaccination as early as possible.
Most of the current batch of vaccines need two doses at an interval of about three weeks to achieve effective immunization. This indicates that a shortage of vaccine supply is bound to occur in the initial phase, irrespective of the context. Secondly, the ability to guarantee that everyone who receives a first dose will turn up for the second is very limited.
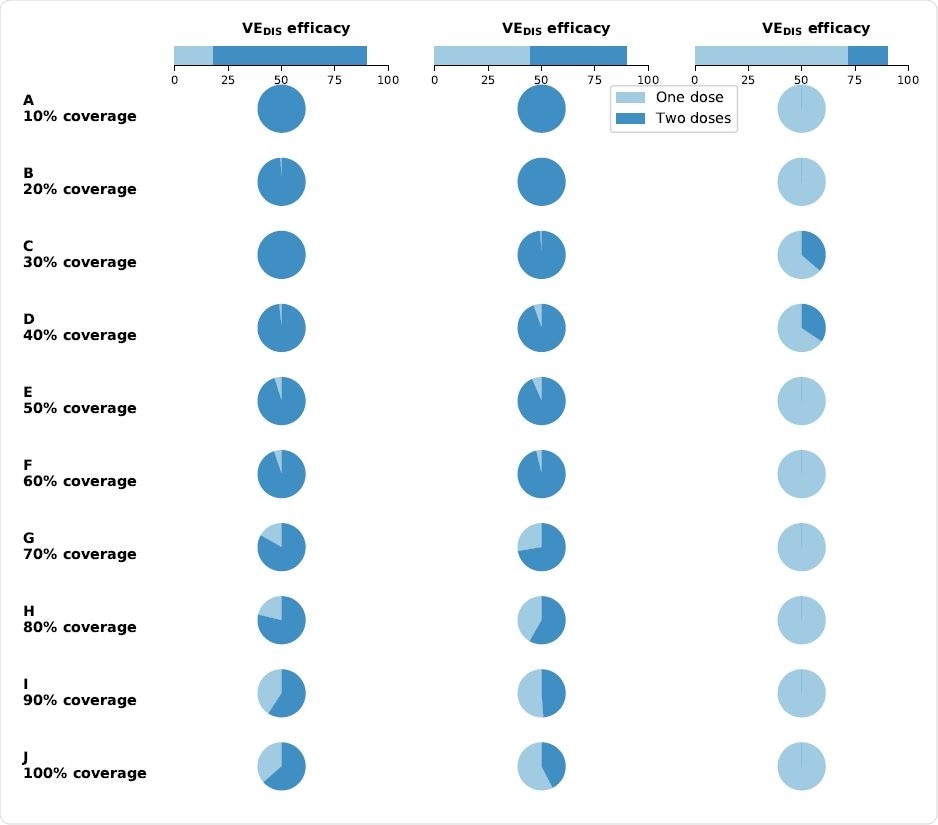
Optimal vaccine allocation as a function of single-dose use and two-dose use. Percentage of total vaccine available utilized for single-dose allocation (light blue) and two-dose allocation (dark blue) when there is enough vaccine to cover 10% (row A) to 100% (row J) of the population with a single dose. Each column of the plot assumes that the efficacy after the first dose of vaccine is low (left column, VEDIS1 = 18%), moderate (center column, VEDIS1 = 45%) or high (right column, VEDIS1 = 72%). Image Credit: Matrajt, L. et al.
The current study looks back to the use of fractional dosing in earlier disease outbreaks. Fractional dosing refers to the use of fewer than recommended doses to stretch out the vaccine supply and has been adopted to prevent yellow fever and cholera. Single-dose vaccination has several advantages, including ease, cost-effectiveness, a more extensive reach, and faster herd immunity.
The critical factor in such a campaign is the efficacy of a single-dose vaccine. The current paper deals with two aspects of vaccination: optimization of vaccine allocation and the number of doses for each recipient. The researchers built on an earlier model of how the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spreads and the mechanics of vaccination against this virus.
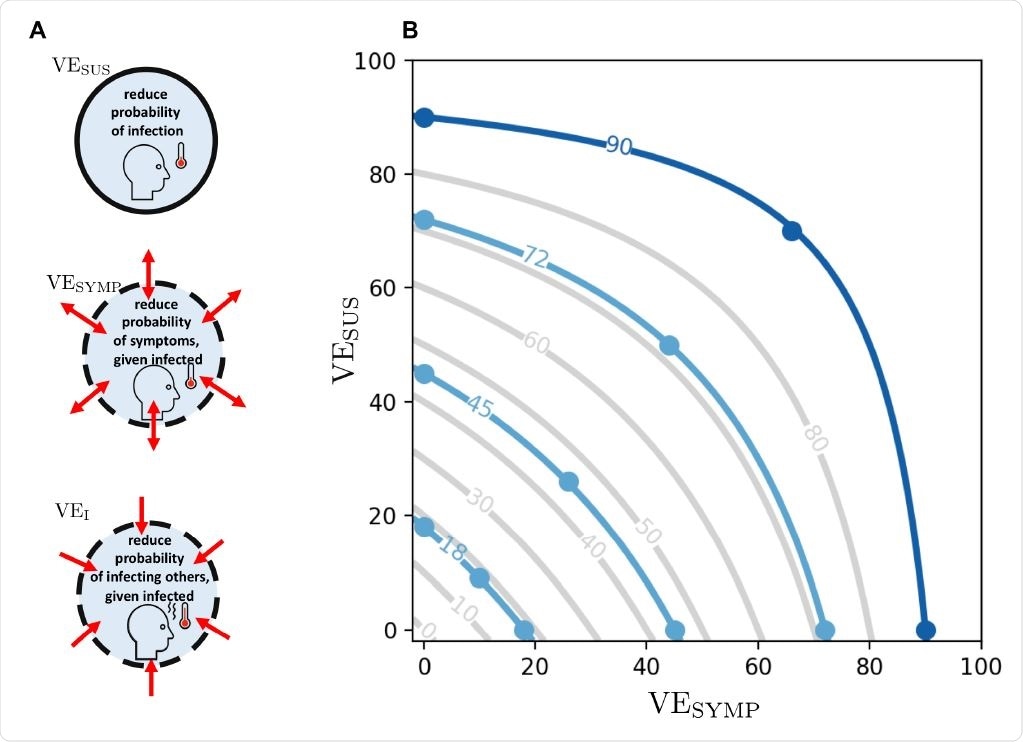
A: Different vaccine effects modeled: a vaccine can reduce the probability of infection, denoted by VESUS. In addition, it can reduce the probability of developing symptoms once infected, denoted VESYMP. Finally, it can reduce the infectiousness of a vaccinated person upon infection, denoted VEI. We assumed that VEDIS can be expressed as a combination of VESUS and VESYMP (see text). B: Level curves for VEDIS as a function of VESUS and VESYMP. The light blue lines indicate the efficacies VEDIS1 obtained after a first dose of vaccine considered in the main analysis. The dark blue line indicates the vaccine efficacy obtained after the full dosage (two doses) VEDIS = 90%. Image Credit: Matrajt, L. et al.
Study details
The study model covered 7.6 million people categorized into 16 groups by age. These were then classified into five vaccination groups. The vaccination campaign they considered was aimed at delivering 3,00,000 vaccine doses a week, with two alternatives –1,50,000 doses a week and instantaneous vaccination. The aim was to cover the whole population by six months with mixed doses. This vaccination speed is fourfold that achieved with influenza vaccination during the 2009 flu pandemic.
The baseline assumptions were that a tenth of the population is immune following natural infection, that both asymptomatic and symptomatic infections are equally infectious, and that both natural and vaccine-induced immunity are long-term. They also considered four levels of social distancing, which led to an effective reproductive number, Rt, from 1.2 to 3.
The researchers placed frontline health and other workers at the top. They also looked at five different metrics, namely, cumulative infections, cumulative symptomatic infections, cumulative deaths, peak hospitalizations (outside the intensive care unit, ICU), and peak ICU hospitalizations.
The researchers considered a leaky vaccine, one which is only partially protective against the vaccines. The effects would include a reduced probability of infection, symptomatic infection, and lower infectivity of vaccinated individuals. They modeled different combinations of these effects in the main scenario, with a single-dose vaccine.
Single-dose efficacy is key
They found that the single-dose efficacy (SDE), vaccine efficacy must be high for an effective single-dose vaccination strategy. With a low SDE and with low vaccine coverage, two doses were almost always required. With increasing vaccine availability, mixed doses were used for optimal coverage.
For high SDE, and with social distancing, the best strategies involve one dose only, whatever the objective. With a medium SDE, mixed doses came to the forefront. For instance, with SDE at 70% or more of total efficacy, all of the vaccines could be allocated proportionally to the population in each age group (pro-rata). Conversely, with low SDE, and 70% coverage with one dose, the optimal schedule would include giving two doses to older adults, using up most of the vaccine.
If SDE is high, an optimal vaccine allocation is exceptionally vital since it could stop almost 50% more deaths compared to a high-risk strategy over six months. Even with low coverage, there was a significant reduction in transmission and in deaths because the vaccine was given to the age group where the most transmission occurred, between 20-50 years, rather than to high-risk but low-transmission older adults.
With 50% coverage and high SDE, optimal allocation strategies could reduce deaths by almost a third compared to the high-risk strategy. In fact, the outbreak could be contained at this coverage, and with this effective reproduction number Reff, even conferring short-term herd immunity. Earlier work shows that with 60% or higher SDE and high coverage, single doses can achieve herd immunity, allowing some normalcy to be restored.
Social distancing and vaccination coverage
At 30% or more coverage, the optimal allocation strategy reduces deaths by 30% to 40% more than high-risk strategies, depending on the SDE. With stringent social distancing and a low transmission rate, the optimal schedule for low SDE vaccines requires two doses for the high-transmission group but pro-rata allocation with high SDE.
The aim was to prevent transmission such that those at high risk are indirectly protected. With high SDE, this optimal allocation would lead to substantial reductions in transmission and up to a 45% reduction in deaths relative to the high-risk strategy.
If the Reff was 1.4, with the same coverage, the high-transmission group was prioritized with one or two doses depending on the SDE. With more coverage, depending on the SDE, the same strategy continues, with pro-rata being preferred if the SDE is very high. If half the population is covered, the optimal allocation can avert 10% to 17% more deaths. Either strategy works well with high vaccine coverage.
At the highest transmission rates, without social distancing, high-risk strategies are preferred at low or medium SDE. Groups are high-priority if SDE is low or medium. With high SDE, a mixed-dose approach is optimal, with one dose for high-transmission groups, and two doses for elderly adults, for a total coverage of 50% of the population. This ensures that the highest-risk individuals are protected directly rather than by herd immunity. In such a situation, vaccination coverage beyond 40% and 60% for effective reproduction numbers of 1.7 and 3, respectively, do not reduce the number of deaths averted further, indicating that the outbreak ended before the conclusion of the vaccination program.
The continuation of strict social distancing allowed for more deaths to be prevented during the vaccination campaign, especially with the limited initial availability of vaccines. In such a situation, the SDE decides the strategy, in conjunction with the rate of vaccination.
“If the SDE is high, slow vaccination campaigns favored the pro-rata strategy, while fast vaccination campaigns favored vaccinating the high-transmission groups with one dose.”
Without social distancing, high-risk strategies become necessary, whatever the vaccination rate.
Mode of action
The mode of action of the vaccine also affects the success of the pro-rata strategy severely at low SDE levels, if only symptomatic disease is prevented, but results in high levels of protection if the SDE is high. The high-risk strategy is not affected at any SDE with this mode of action.
Implications
It is absolutely necessary to understand SDE of available vaccines in order to use them optimally to end the pandemic as rapidly as possible. With the additional availability of vaccines, more single doses could be offered to increase vaccine coverage. In addition, social distancing is essential to protect the population until adequate coverage is attained.
“In agreement with others, our results show the absolute necessity to keep social distancing interventions in place: if social distancing interventions are lax, or if the vaccine is not rolled out fast enough, then our results show that the effect of vaccination will be limited, and that the current wave of infections will be over long before.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources