As the coronavirus disease 2019 (COVID-19) pandemic breaks out afresh over much of the world, vaccines are assuming greater significance. Several types of vaccines have been or are in the process of being developed against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19. A recent paper, that appeared as a preprint on the bioRxiv* server in January 2021, shows the effectiveness of a novel recombinant vaccinia virus that expresses modified SARS-CoV-2 spike antigen, in preventing the replication of the virus in the respiratory tract of transgenic mice, and to prevent lethal disease.
The replication-restricted modified vaccinia virus Ankara (MVA) was used to create a licensed smallpox vaccine, as well as in many past and present studies that use recombinant MVAs (rMVAs) as viral vectors to prevent other infectious diseases. Currently, early studies are ongoing that use rMVA to protect against COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The spike (S) protein is the major target of neutralizing antibodies. The current study used a set of recombinant MVAs that expressed either wild-type or modified S protein. The S protein modifications included two proline substitutions, mutant furin cleavage site, and deletion of the endoplasmic retrieval signal.
High spike expression
In the first part of the study, involving cell cultures, they found that with all rMVAs, there were almost equivalent levels of expression of the spike protein on the surface of the rMVA-infected cell. The different rMVAs also elicited similar titers of neutralizing antibodies that inhibited a spike-bearing pseudovirus. All the rMVAs expressed the receptor-binding domain (RBD) on the cell surface, as indicated by the reaction of the cells with anti-RBD mAb and soluble hACE2.
The rMVAs elicited a strong predominantly Th1 type immune response with IgG2a or IgG2c being the initial antibody type, followed by IgG2b, IgG1, and finally IgG3. This is the usual response to a viral infection, and is caused by interferon-gamma stimulation. IgG2a, IgG2b and IgG2c all fix complement and activate Fc receptors, to enhance the clearance of virus from the body. IgG1 may reduce inflammation.
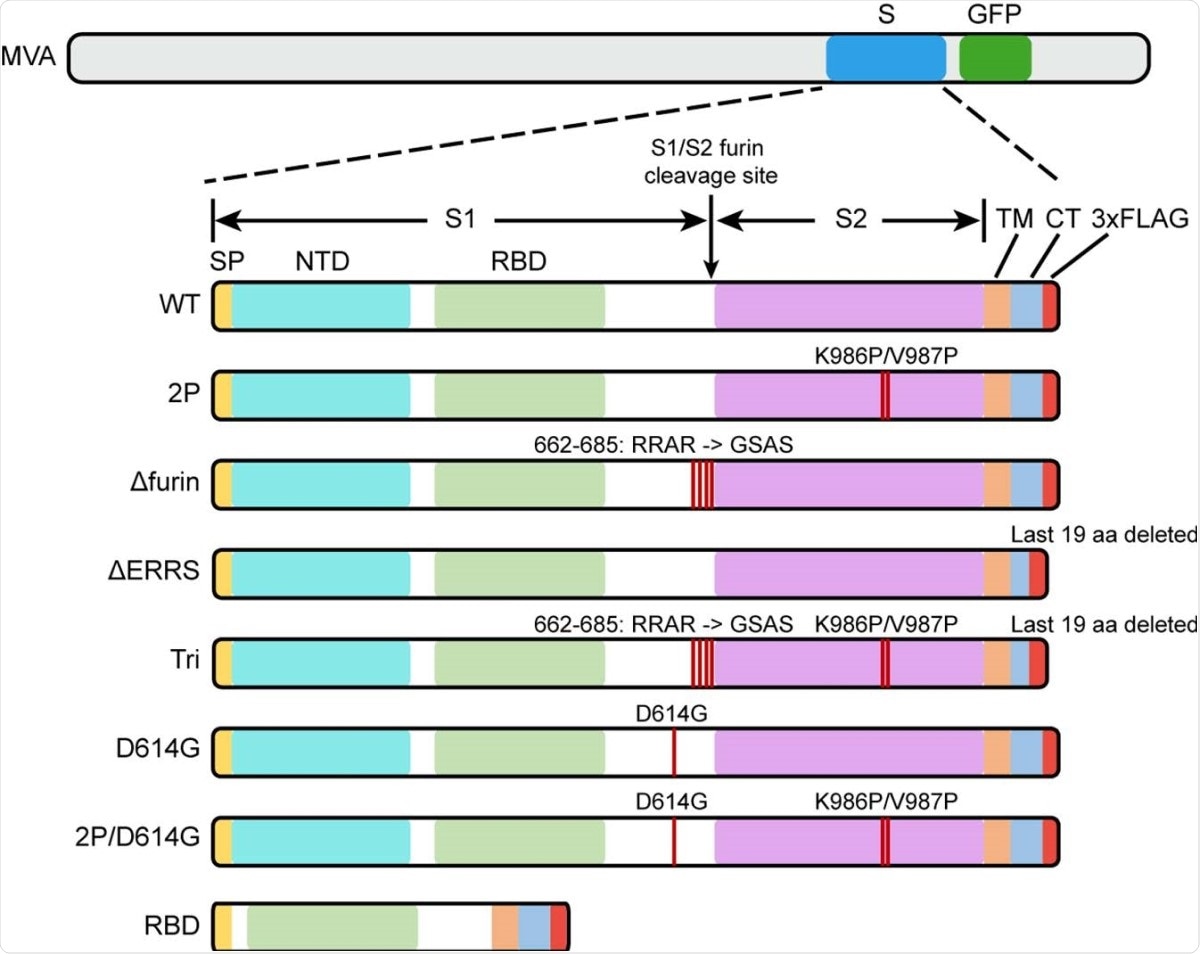
Diagrams of rMVAs. Top shows approximate locations of CoV-2 spike protein (S) and green fluorescent protein (GFP) ORFs within rMVA. Modifications of S ORF are shown below with names of constructs on the left. Abbreviations: SP, signal peptide; NTD, N-Terminal domain; TM, transmembrane domain; CT, C-terminal domain; RBD, receptor binding domain; 3xFLAG, 3 tandem copies of FLAG epitope tag.
Binding and neutralizing antibodies
The researchers experimented with various prime-boost regimens, and found that the use of purified adjuvanted RBD led to lower levels of binding and neutralizing antibodies. In mice, this was associated with a predominant IgG1 response. When rMVA priming was followed by RBD boosting, higher antibody titers resulted, both binding and neutralizing, compared to homologous rMVA prime-boost regimens.
This could be because the booster dose of the virus is inactivated or weakened by the immune response to the live virus vector formed in response to the priming dose. A longer interval could overcome this attenuation effect.
With an rMVA prime and RBD boost, IgG2a and IgG2c predominance was maintained, in keeping with an anamnestic Th1-type response. With this regimen, the CD8+IFNg+ T cell response was lower, however. An rMVA boost may be required to maintain high levels of S-specific T cells.
Protection against viral challenge
In mouse immunization studies, the titer of neutralizing antibody was acceptable relative to that achieved by mRNA immunization. Further study will be required to understand the immune response in other animal models and in humans.
Transgenic mice expressing human angiotensin-converting enzyme 2 (hACE2) were used to assay protection against SARS-CoV-2 infection following immunization with rMVAs. The viral challenge was given either after the prime-boost course, or after priming alone. In the former case, both homologous and heterologous rMVA regimens were followed.
The immunized mice showed no signs of illness following viral exposure, while controls lost weight and became sick in 5-6 days after intranasal inoculation with the virus. The immunized mice remained healthy even after a second exposure to the virus after two weeks.
While control mice had high viral loads in the lungs on day 2, with only a small drop by day 5, no virus was seen in the lungs of any immunized mouse. Again, the virus was isolated from the nose of all controls, but from only one of the vaccinated mice on day 2, at low titer, and none on day 5, indicating abortive replication.
Viral subgenomic RNAs were found at low levels in the nose of all vaccinated mice on day 2, and cleared by day 5. This may mean that local immunization is necessary to provide sterilizing immunity, which systemic vaccination with rMVA was unable to do. Only traces of the nucleocapsid (N) mRNA were found in some vaccinated mice, on day 2, and no spike (S) mRNA, but both were found at significant levels in the lungs of control mice.
Mice vaccinated with one dose of rMVA were also protected, with no detectable virus or subgenomic RNA in their lung or nasal tissue on day 5.
Passive immunity transfer
Finally, the possibility of passive transfer of immunity was explored. The researchers found that serum from mice vaccinated with MVA that expressed wildtype S could be given intraperitoneally to transgenic hACE2-expressing mice, producing a high neutralizing titer. When these passively immunized mice were then exposed to a viral challenge, they remained healthy. This shows that this vaccine could elicit sufficient levels of protective antibodies and that the neutralizing titers induced by this immunization exceed the required level significantly.
Implications
In a transgenic mouse model, one or two injections of recombinant MVAs that express modified forms of S inhibited CoV-2 replication in the upper and lower respiratory tracts … prevented severe disease.”
The rMVA vaccine is temperature-stable, which makes its logistics favorable. It can remain stable at 2-8oC for a year, and can be frozen for up to two years. It can be given subcutaneously, intramuscularly or orally, as well as by the intranasal route or by aerosol. This raises the prospect that it could be used intranasally to prevent viral replication altogether, beyond the rapid and significant reduction in viral replication within the nose of vaccinated mice, as seen with intramuscular administration in this experiment.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources