Researchers in Spain have demonstrated that studying the hypoxia signaling pathway could provide opportunities for treating coronavirus disease 2019 (COVID-19).
The team conducted a study showing that hypoxic (oxygen-deficient) conditions reduced the expression of host cell receptors for the causative agent – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Hypoxic conditions reduced expression of angiotensin-converting enzyme 2 (ACE2) – the primary host cell receptor required for viral attachment and entry. They also decreased the expression of heparan sulfate (HS), another receptor known to be required for SARS-CoV-2 attachment.
The team – from the Basque Research and Technology Alliance in Derio and the Basque Foundation for Science in Bilbao also showed that hypoxia decreased HS levels through reduced expression of the main HS-containing proteoglycans (HSPGs) – syndecan-1 and syndecan-3.
“Our study indicates that hypoxia acts to prevent SARS-CoV-2 infection, suggesting that the hypoxia signaling pathway might offer therapeutic opportunities for the treatment of COVID-19,” writes Asis Palazon and colleagues.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
Hypoxia a common feature of COVID-19
A common feature among COVID-19 patients is moderate-to-severe hypoxia, but how hypoxia influences SARS-CoV-2 infectivity and disease pathogenesis is not yet clear.
However, studies have shown that low oxygen levels might reduce SARS-CoV-2 infectivity and disease severity.
The SARS-CoV-2 infection process involves the receptor-binding domain (RBD) within subunit 1 (S1) of a viral surface protein called a spike. This RBD interacts with the host cell receptors ACE2 and HS before subunit 2 (S2) of spike mediates fusion between the virus and cell membrane.
Research has shown that ACE2 expression is regulated by hypoxia in a time-dependent manner and epidemiological data suggest that COVID-19 severity decreases in high altitude areas.
Furthermore, it has also been demonstrated that syndecans – the main HSPGs – are differentially regulated by hypoxia.
“Our hypothesis is that hypoxia influences the cellular attachment of SARS-CoV-2 into host cells by modulating the expression of viral entry and attachment receptors on epithelial cells,” says Palazon and the team.
What did the researchers do?
To investigate whether hypoxia affects cellular binding of the spike protein, the researchers subjected human lung epithelial cells (NCI-H460) and Vero E6 cells incubated with RBD and S1 to different oxygen concentrations (21% versus 1% oxygen).
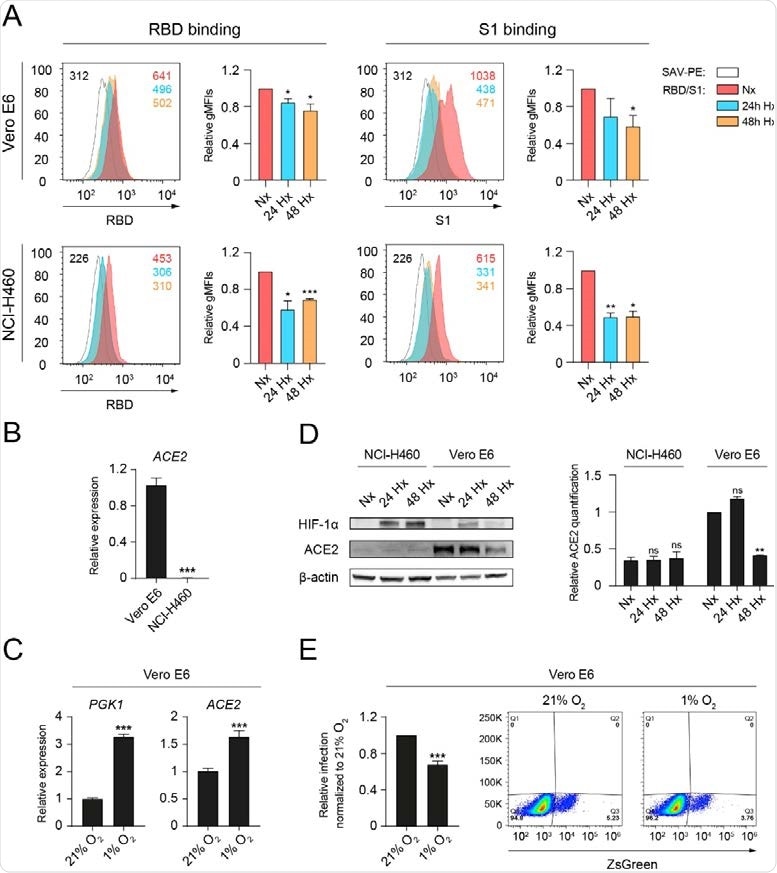
Hypoxia reduces the binding of RBD and S1 to epithelial cells and decreases ACE2 protein levels. (A) Binding of the receptor binding domain (RBD) (left) or S1 subunit (S1) (right) to Vero E6 (top, n=3, unpaired t test) or NCI-H460 (bottom, n=2, unpaired t test) cells cultured under normoxia (21% oxygen) or hypoxia (1% oxygen) for 24 and 48 hours, measured by flow cytometry. A representative histogram indicating the geometric mean fluorescent intensity (gMFI) value for each condition is shown. (B) Relative ACE2 gene expression on Vero E6 and NCI-H460 measured by Q-PCR (n=3, unpaired t test). (C) Relative gene expression of PGK1 (left) and ACE2 (right) on Vero E6 cells cultured under normoxia or hypoxia for 24 hours (n=3, unpaired t test). (D) (Left) Western blot of HIF-1α, ACE2 and β-actin on Vero E6 and NCI95 H460 cells cultured under normoxia or hypoxia for the indicated time points. (Right) Relative quantification of ACE2 protein expression by densitometry (n=2, 2-way ANOVA). (E) Relative infection of Vero E6 cells with pseudotyped viral particles expressing the spike protein of SARS98 CoV-2, measured by flow cytometry based on ZsGreen expression (n=4 independent experiments). Error bars represent SEM. Asterisks represent p values (*, ≤ 0.05; **, < 0.01; ***, < 0.001).
Under hypoxic conditions (1% oxygen), binding of RBD and S1 was significantly reduced in both cell lines.
The team assessed ACE2 gene expression using quantitative PCR (polymerase chain reaction) to explore the mechanism underlying this reduced binding.
This revealed that only the Vero E6 cells presented detectable levels of ACE2. While hypoxia significantly increased ACE2 transcription in Vero E6 cells, it significantly reduced the total levels of ACE2 protein.
The researchers say this might at least partially explain the reduced cellular binding of RBD and S1.
They showed that Vero E6 cells cultured under hypoxic conditions exhibited a decreased infection rate with pseudotyped SARS-CoV-2 lentiviral particles.
“The observed reduction of ACE2 protein levels contrasts with the early transcriptional increase of this gene,” says the team.
However, this likely results from the binding of the hypoxia-inducible factor (HIF) transcription factors to hypoxia response elements on the ACE2 promoter, they add.
“The observed discrepancy at the RNA versus protein ACE2 levels might be explained by protein degradation, cleavage, or time-dependent adaptation to hypoxia.”
What about the NCI-H460 cell line?
The NCI-H460 cell line contains significantly less ACE2 transcripts compared with Vero E6 cells, and does not express detectable levels of ACE2 protein.
However, the interaction of the RBD and S1 with these cells was still strongly inhibited by hypoxia, indicating the presence of additional SARS-CoV-2 binding factors that are regulated by hypoxia.
Next, the team explored whether levels of the host cell receptor HS were altered under hypoxic conditions.
This revealed that the total level of HS expressed on both NCI-H460 and Vero E6 cells were significantly decreased under hypoxic conditions.
The researchers then used flow cytometry to assess the expression of syndecans and found that syndecan-1 and syndecan-3 were the most abundant on both cell lines.
The study revealed that hypoxia downregulated levels of syndecan-1 on NCI-H460 cells and levels of syndecan-3 on Vero E6 cells.
Palazon and colleagues say the study shows that hypoxia reduces the SARS-CoV-2 binding of host cells by at least two different mechanisms: hypoxia decreases levels of the viral entry receptor ACE2 and levels of HS within cell surface HSPGs.
“The significance of these findings at physiopathological oxygen levels remains unexplored,” write the researchers.
“In this context, elucidating the role of the HIF signaling pathway might unlock novel therapeutic targets that, when modulated, reduce the initial virus-host interaction and viral load,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Palazon A, et al. Hypoxia reduces cell attachment of SARS-CoV-2 spike protein by modulating the expression of ACE2 and heparan sulfate. bioRxiv, 2020. doi: https://doi.org/10.1101/2021.01.09.426021, https://www.biorxiv.org/content/10.1101/2021.01.09.426021v1
- Peer reviewed and published scientific report.
Prieto-Fernández, Endika, Leire Egia-Mendikute, Laura Vila-Vecilla, Alexandre Bosch, Adrián Barreira-Manrique, So Young Lee, Ana García-del Río, et al. 2021. “Hypoxia Reduces Cell Attachment of SARS-CoV-2 Spike Protein by Modulating the Expression of ACE2, Neuropilin-1, Syndecan-1 and Cellular Heparan Sulfate.” Emerging Microbes & Infections 10 (1): 1065–76. https://doi.org/10.1080/22221751.2021.1932607. https://www.tandfonline.com/doi/full/10.1080/22221751.2021.1932607.