While the coronavirus disease 2019 (COVID-19) pandemic continues to wreak havoc in many parts of the world, a new variant of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged.
This new variant, called N501Y, has caused skyrocketing cases in the UK, where it first emerged in September 2020. To date, the country has reported more than 3.12 million cases and over 82,000 deaths.
The rise of this highly infectious new strain has raised serious questions about whether or not the current vaccine candidates will be effective in immunizing against it. It has also brought a new urgency to the vaccination effort in the UK and elsewhere where roll-outs have begun.
Researchers from Pfizer and the University of Texas, USA, have presented evidence showing that people in a previously reported trial of the mRNA-based COVID-19 vaccine, BNT162b2 – commonly known as the Pfizer-BioNTech COVID-19 vaccine – had equivalent neutralizing titers to the N501 and Y501 viruses, suggesting that the vaccine could be effective against this new strain. The team's findings have been published on the preprint bioRxiv* server.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Fast spreading SARS-CoV-2 variant
Emerging in September, a new strain of SARS-CoV-2 has recently exploded across southeast England, prompting the government to impose stringent restrictions and lockdown orders in what is now a third national lockdown since the pandemic first begun. The new variant is highly transmissible and fast-spreading.
The new variant contains 23 mutations, 8 of which are in the spike proteins, the part of the virus that binds human cells and facilitates its entry. The novel mutations include the N501Y mutation that allows the virus to bind more tightly to the angiotensin-converting enzyme 2 (ACE2) receptor. It also contains the D614G deletion, which has been observed to spread more rapidly between people than earlier strains.
In an investigation of the new strain conducted by Public Health England, researchers have noted that the N501Y shows significantly higher transmissibility than previous strains, though have not found any evidence to suggest it is more lethal or virulent.
The study
In the study, the researchers generated isogenic N501 and Y501 SARS-CoV-2. Blood samples of participants in a previously reported trial of the BNT162b2 vaccine showed equivalent neutralizing titers to the new variants.
To arrive at the study findings, the researchers obtained the blood samples from the participants that were obtained 2 or 4 weeks after immunization with two 30- µg doses of BNT162b2 spaced three weeks apart. The blood samples were tested for neutralization assay.
The results showed that the ratio of the 50 percent neutralization geometric mean titers (GMTs) of the blood samples against the Y501 virus to that against the N501 virus was 1.46. This means that there was no reduction in the neutralization activity against the SARS-CoV-2 that contains the Y501 spike.
Nevertheless, preserved neutralization of Y501 virus by BNT162b2-elicited human sera is consistent with preserved neutralization of a panel of 15 pseudoviruses bearing spikes with other mutations found in circulating SARS-CoV-2 strains,” the researchers concluded in the study.
The Pfizer-BioNtech vaccine has demonstrated a 95 percent efficacy in protecting against COVID-19 in its human trials, which involved at least 44,000 participants who are 16 years of age or older. The results present evidence that the vaccine could potentially target even the novel variants, which have caused surging cases in some countries, particularly in the UK and South Africa.
Since SARS-CoV-2 is a novel virus, it is expected to evolve, leading to the emergence of new variants. The ongoing evolution of the virus requires persistent monitoring of the significance of changes to ensure vaccine coverage. Many scientists have emphasized the importance of preparing for future mutations of the virus, which may necessitate a vaccine strain change.
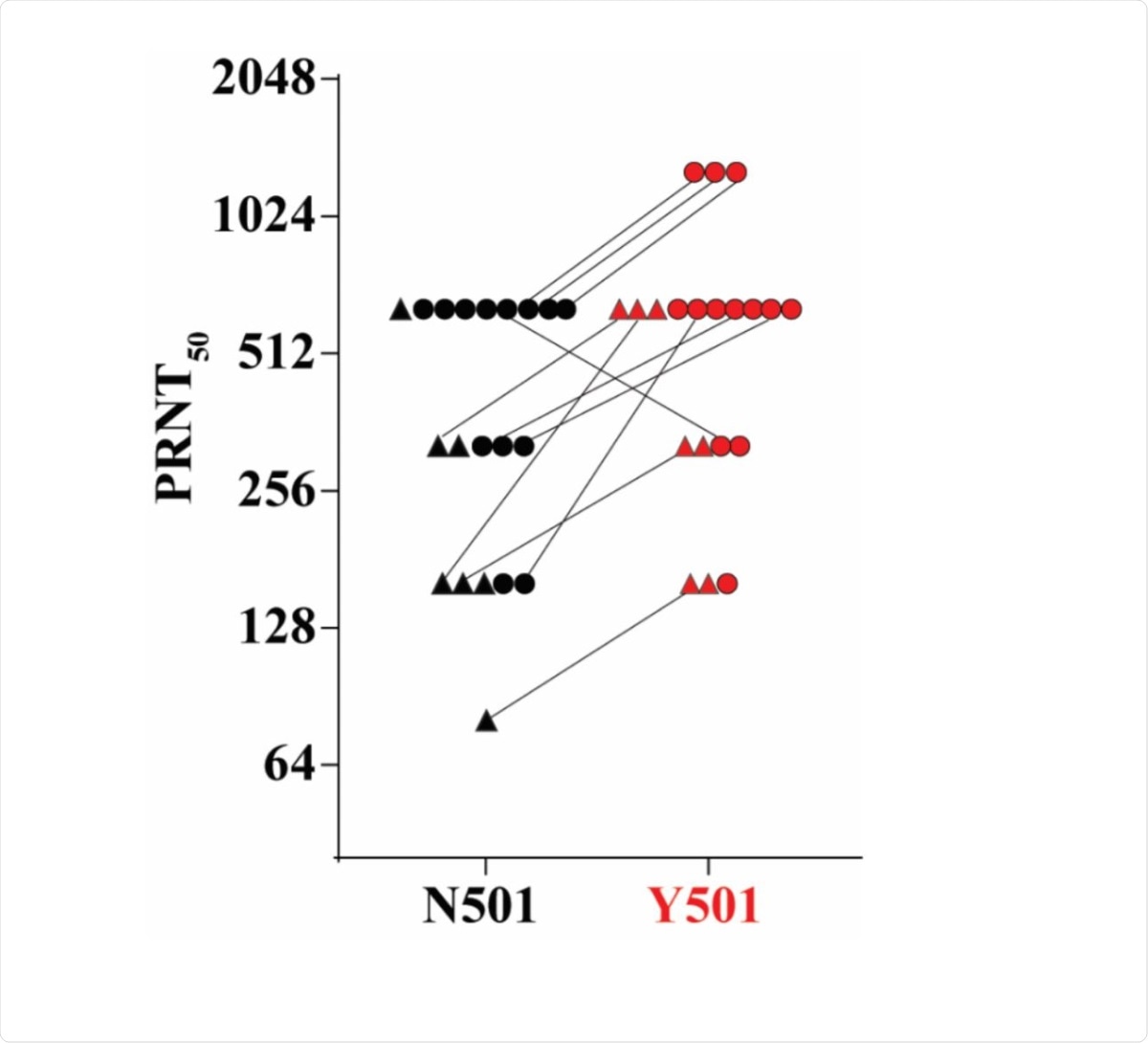
50% plaque reduction neutralization titers of 20 sera from BNT162b2 vaccine recipients against N501 and Y501 SARS-CoV-2. Seven sera (indicated by triangles) were drawn 2 weeks after the second dose of vaccine; 13 sera (indicated by circles) were drawn 4 weeks after the second dose.
A previous study has also noted that it is better to target fast-spreading strains of the virus to reduce the initial ballooning of cases.
To date, there are nearly 91 million confirmed COVID-19 cases. Of these, 1.94 million have died from the infection. The United States reports the highest number of cases, reaching 22.61 million; followed by India, with 10.47 million cases; Brazil, with about 8.13 million cases; and Russia, with over 3.41 million cases. The virus has now reached 191 countries and regions across the globe.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Sources:
Journal references:
- Preliminary scientific report.
Xie, X., Zou, J., Fontes-Garfias, C., et al. (2021). Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. bioRxiv. doi: https://doi.org/10.1101/2021.01.07.425740, https://www.biorxiv.org/content/10.1101/2021.01.07.425740v1
- Peer reviewed and published scientific report.
Xie, Xuping, Yang Liu, Jianying Liu, Xianwen Zhang, Jing Zou, Camila R. Fontes-Garfias, Hongjie Xia, et al. 2021. “Neutralization of SARS-CoV-2 Spike 69/70 Deletion, E484K and N501Y Variants by BNT162b2 Vaccine-Elicited Sera.” Nature Medicine, February, 1–2. https://doi.org/10.1038/s41591-021-01270-4. https://www.nature.com/articles/s41591-021-01270-4.