The potentially deadly coronavirus disease 2019 (COVID-19) that has swept the world over the last 12 months has not affected animals with corresponding severity. In order to understand the mechanism of severe disease, animal models have been used. This includes a mouse model that expresses human angiotensin-converting enzyme 2 (hACE2) under the cytokeratin 18 promoter (K18-hACE2). A new preprint appearing on the bioRxiv* server indicates that this may not be a faithful model of human lethal infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
In most cases, COVID-19 causes respiratory disease with a wide spectrum of symptom severity, from mild to severe. Critical disease usually terminates in acute respiratory distress syndrome (ARDS), often with multi-organ dysfunction. A subset of infected patients also have neurological features, including headache, anosmia and ageusia, dizziness, delirium, and ischemic stroke. The olfactory mucosa has been thought to be the port of entry for the virus into the central nervous system (CNS).
Animal models that help to study the virus have been few. This is mainly because SARS-CoV-2 enters human cells via the hACE2 receptor. On the other hand, the virus has a much lower affinity for murine ACE2, making transgenic mice a necessity for preclinical research.
These transgenic mice have been observed to express 100% lethal disease when infected. The mechanism by which death occurs is as yet unknown, though there were signs of lung inflammation and reduced respiratory function. However, the virus has been reported to invade the central nervous system in these mice.
The investigators in the current study therefore analyzed the course of the infection for two weeks following the infection. The expression of hACE2 is mainly in the epithelial cells in the respiratory and intestinal tracts, with a low level of expression in the brain. The scientists therefore aimed at exploring the relationship between the nature, severity and outcome of this infection in these transgenic mice and hACE2 expression patterns, in order to find out if the neuroinvasion was related to the lethal nature of the infection.
SARS-CoV-2 infection is always fatal in these mice
The researchers found that these mice always developed a lethal infection following SARS-CoV-2. Before death, they had neurological signs. Only two mice were alive at the end of 14 days, both of which were not seriously sick.
These mice were seen to have inflamed lungs, with high viral loads in both the lungs and the brain. The viral load in the lungs peaked at 2 days post-infection (dpi), but at 4 dpi in the brain. Viremia was negligible.
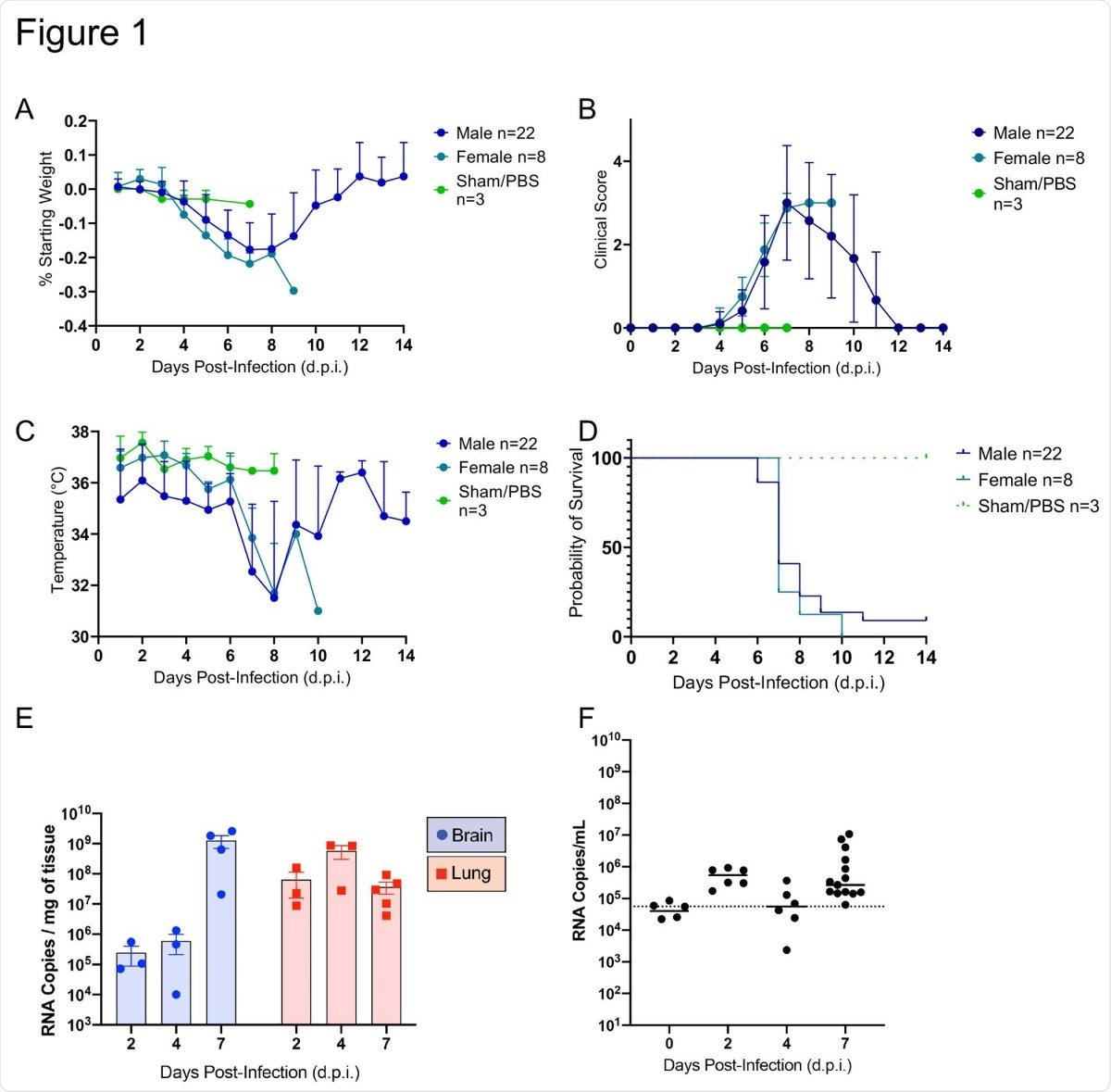
SARS-CoV-2 caused lethal disease in K18-hACE2 mice (n=33) inoculated intranasally with 1 x 106 plaque forming units (PFU). Body weight (A), clinical signs (B), temperature (C), and mortality (D) were monitored daily. Viral loads (genome copy numbers/mg or ml) were monitored in the brain, lungs (E) and serum (F) throughout the study. Mean genome copy numbers are depicted. The limit of detection (LOD) is shown with a dashed line.
Transient nasal infection
The upper respiratory tract was found to have signs of mild and transient inflammation, reverting to normal at 7 dpi. Olfactory neuroepithelium was not found to show any signs of inflammation at any point, despite the presence of abundant viral antigenic load and RNA. However, both declined to undetectable levels by 14 dpi.
Moderate interstitial pneumonia
The study also showed some degree of interstitial pneumonia, with both viral antigens and RNA being found in the lung parenchyma, within type I alveolar lung cells for the most part. A few type II alveolar cells were also involved. The peak was reached at 4 dpi. Pneumonia severity peaked at 7 dpi, involving about a third of the lungs.
In the two male rats who survived at 14 days, there were scattered areas of interstitial pneumonia, but no detectable viral antigen or RNA.
Bronchial epithelium and lung vasculature remained unaffected throughout, with no hyaline membrane formation in the lungs, neither vascular clots or syncytia formation. These are characteristic of human infection, however. The features of lung involvement seen in these animals contrast with the disease characteristics of severe COVID-19. This suggests that “the lethality observed in this model might be independent of lung inflammatory mechanisms.” Indeed, the strong infiltrating response by lymphocytes and histiocytes was associated with rapid and effective control of infection in the lungs.
Lethality associated with neuroinvasion
The researchers found that SARS-CoV-2 lethality is potentially mediated by neuroinvasion, in about 85% of infected mice, at 7 dpi. The probable route of invasion is by retrograde transport through the olfactory bulb, via the axonal processes passing through the olfactory neuroepithelium. The affected neurons showed a widespread cytopathic effect in both the brain and the spinal cord.
SARS-CoV-2 infection does not mirror ACE2 distribution
The study also shows that the lungs poorly express hACE2 in these transgenic mice, which indicates that this is not the only factor determining susceptibility to the virus. Conversely, other cell types that do not express this receptor, such as type I alveolar cells, were widely infected, but hACE2+ bronchial epithelial cells remained uninfected throughout. This suggests that viral entry into lung cells is not dependent on ACE2 expression, in these mice.
The researchers also found that while hACE2 mRNA was found in cortical neuron clusters and other parts of the brain, murine ACE2 was expressed in the endothelium of the brain’s blood vessels. This suggests that ACE2 may not be translated in neurons in mice. The Purkinje cells of the cerebellum are also not infected by SARS-CoV-2, though they do express hACE2 mRNA. This confirms the hypothesis that “ACE2 is likely not the sole host factor associated with neuroinvasion and that other ACE2-independent entry mechanisms contribute to neuroinvasion and spread by SARS-CoV-2 in this murine model.”
What are the implications?
The study throws up a few important findings. Firstly, the K18-hACE2 transgenic mouse model is widely used for the study of SARS-CoV-2 and for potential therapeutic measures. The basis for such use is the common hACE2 receptor shared by cells of this mouse and humans, as well as the lethal expression of SARS-CoV-2. It also helped to understand how lung injury occurred in COVID-19.
However, it also diverges from the human COVID-19 disease process in several important ways. It does not show the development of diffuse alveolar damage (DAD), hyaline membrane formation, activation of the coagulation system with extensive microthrombosis, resulting in multi-system damage.
In the mouse model, instead, hACE2 is only partially associated with the tropism of the virus and the disease outcomes. The real cause of lethal outcomes is neuroinvasion leading to damage to the neurons, which is independent of hACE2 expression. This is in contrast to golden Syrian hamsters, which suffer primarily pulmonary lesions but invariably survive.
The study also points to the need to select the right endpoints to understand the neurological features of SARS-CoV-2 in mice, rather than the conventional features such as weight loss and ruffled fur suggestive of sick animals. If not, the efficacy of therapeutic drugs and T cell-based vaccines might be falsely underestimated in this model.
Finally, the study points to the existence of other receptors that mediate neuroinvasion, which cannot be identified by in vitro experiments. One such is neuropilin-1, found in the nose, brain, lungs, liver and kidneys. Since K18-hACE2 mice are among the only two animal models that show this neuroinvasion, it is useful for the study of neurologic disease processes following infection by this virus, including the other mechanisms by which it accomplishes cell entry, and for estimating the efficacy of therapeutic and preventive measures.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources