Especially among the elderly, community-acquired pneumonia (CAP) is a major cause of death. The current coronavirus disease 2019 (COVID-19) pandemic has brought the focus on multiple common treatment practices for hospitalized COVID-19 patients, including empirical antibiotic therapy for suspected bacterial superinfection. However, a new study by researchers at Northwestern University Feinberg School of Medicine, USA, shows that this may be promoting an unnecessarily high incidence of antibiotic usage. The team’s findings are available on the medRxiv* preprint server.
The poor results with the attempted culture of bacteria from patients who have severe viral pneumonia has made it difficult to evaluate the value of antibiotic therapy in this condition. Now, molecular diagnosis has made it easier to detect respiratory pathogens, making it possible to stop antibiotics in patients being treated for severe CAP, even those on mechanical ventilation.
BALF offers better results
Samples for such testing should be collected from bronchoalveolar lavage fluid (BALF), rather than from nasopharyngeal swab or endotracheal aspiration, for better results. This procedure is not known to be safe in COVID-19 patients, however, and is thus in limited use. This means that the actual prevalence of bacterial superinfection in patients with COVID-19 pneumonia, as well as of the development of ventilator-associated pneumonia (VAP) in these patients, remains unknown.
The researchers used BALF in patients who were intubated for respiratory failure as a result of COVID-19 pneumonia. The data was taken from a cohort of 196 patients treated during the first wave of COVID-19, from March 1 to June 30, 2020. Some of these patients (28%) were from another hospital, and were more likely to receive extracorporeal membrane oxygenation (ECMO), to have a higher mortality, and to be on mechanical ventilation for a shorter period.
CAP-like pathogens
About 75% of patients had a BALF sample taken within 48 hours of intubation, though this was less likely in patients transferred in from outside, especially since most of the latter remained on mechanical ventilation less than two days. About 28% of this group remained in hospital for over 48 hours, and about one in five had bacterial superinfection. Thus, this led to a definition of suspected hospital-acquired pneumonia (HAP).
The causative pathogens were those typically found in CAP, including Streptococcus species and methicillin-susceptible S. aureus (MSSA). In fact, these two species made up almost 80% of cases. Three patients showed the presence of bacteria resistant to standard CAP antibiotics, two of them being multi-drug resistant S. aureus (MRSA). That is, they required treatment with a carbapenem antibiotic, for Gram-negative bacteria, or vancomycin or linezolid for S. aureus.
There was no clinical parameter capable of differentiating patients with or without bacterial superinfection. However, in patients with SARS-CoV-2 pneumonia, BALF composition differed significantly from that caused by other respiratory pathogens. Nonetheless, no characteristic discriminatory pattern was observed in SARS-CoV-2 patients with superinfection.
Median antibiotic usage
The researchers also assessed the antibacterial therapy using Narrow Antibiotic Treatment (NAT) scores, based on whether they received standard treatment with ceftriaxone-azithromycin in combination, or only one of the drugs, or neither, or more than these two drugs. Median daily NAT scores ranged from -1 in the first seven days, corresponding to the use of a single drug. In those with a positive and negative BALF, the median scores were -1 and -1.5, respectively. There was a median difference in NAT scores between these two groups, of -1.
VAP incidence
In the group, 162 patients were intubated for over 48 hours, and 246 repeat BALF samples were taken from them after this period, thus excluding only a tenth of these patients from multiple BALF sampling. Most of the latter spent less time on the ventilation, with a median of 5 days, compared to the rest, who had a median duration of 14 days.
The researchers found that 72 of these patients had a diagnosis of VAP, accounting for over 44% of the group. The earliest incident occurred about 11 days after intubation, and among these patients, a fifth had another incident of VAP at 10 days from the first. Three patients had a third VAP. In 30 BAL samples, VAP was found to be due to the same bacteria as the earlier VAP. The incidence of repeated VAPs tended to be higher in those who had early superinfections, or MDR infections. MDR infections occurred in a fifth of initial VAPs, with ~78% being caused by a single bacterium.
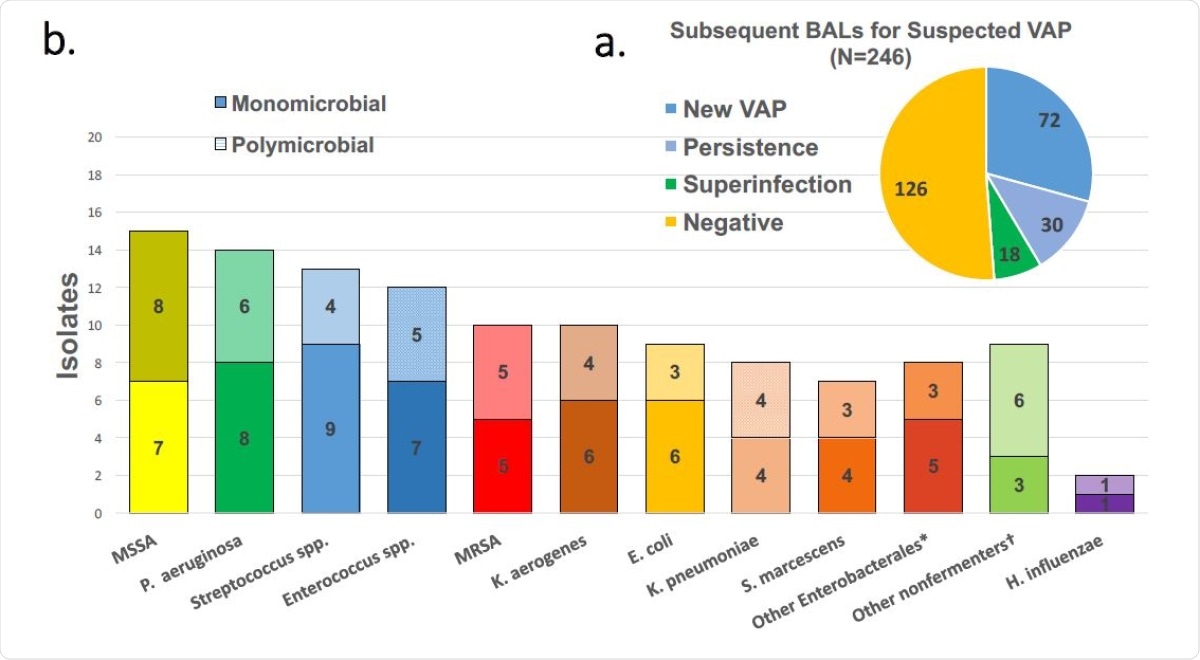
BAL results for suspected VAP. (a) and pathogens detected in positive BALs (b). Solid bars are pathogens detected in monomicrobial episodes while crosshatched are presence in polymicrobial pneumonias.
Overall, there were about 45 VAPs per 1,000 days of mechanical ventilation, showing a linear trend with the number of days until there were very few patients left on the ventilator.
Outcomes
Overall, about a fifth of patients in hospital died, with the odds being almost three times higher among those transferred in from outside. The presence of initial bacterial superinfection was associated with longer ventilation and the need for tracheostomy and chronic respiratory assistance, than with higher mortality.
What are the implications?
The findings that only a fifth of patients with SARS-CoV-2 pneumonia had bacterial superinfection indicates that the current empirical antibiotic guidelines would lead to an excessive use of antibiotics in this patient group. The use of more sensitive and accurate BALF analysis allowed fewer and more focused antibiotics to be used, which is important since most of the (admittedly frequent) VAPs were not due to MDR bacteria.
Despite the high prevalence of HAP in this cohort, most isolates were from those typically found in CAP, especially Streptococcus species and MSSA, even in early VAP detected at 3-7 days following intubation. Taken along with the earlier finding, it appears that “most patients intubated with SARS-CoV-2 pneumonia do not require antibiotics and those that do, can often be managed with narrow-spectrum therapy.”
Again, this may answer the question as to why early antibiotic administration does not significantly reduce the death rate in COVID-19 pneumonia. The researchers comment on their practice of discontinuing antibiotics following a negative BALF result: “Any clinical benefit of greater BAL diagnostic accuracy is dependent on clinical confidence in the results sufficient to not initiate, narrow or discontinue empirical antibiotic therapy; ignoring results (continuing empirical antibiotic management) will not result in benefit.” Following up on these results can prevent substantial antibiotic overuse in such patients.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Pickens, C. O. et al. (2021). Bacterial superinfection pneumonia in SARS-CoV-2 respiratory failure. medRxiv preprint. doi: https://doi.org/10.1101/2021.01.12.20248588, https://www.medrxiv.org/content/10.1101/2021.01.12.20248588v1
- Peer reviewed and published scientific report.
Pickens, Chiagozie O., Catherine A. Gao, Michael J. Cuttica, Sean B. Smith, Lorenzo L. Pesce, Rogan A. Grant, Mengjia Kang, et al. 2021. “Bacterial Superinfection Pneumonia in Patients Mechanically Ventilated for COVID-19 Pneumonia.” American Journal of Respiratory and Critical Care Medicine 204 (8): 921–32. https://doi.org/10.1164/rccm.202106-1354oc. https://www.atsjournals.org/doi/10.1164/rccm.202106-1354OC.