A team of researchers has immunoengineered a nanobead system for the isolation and detection of circulating tumor cells (CTCs). A report on their research has been released on the bioRxiv* preprint server.
Cancerous tissues shed free tumor cells – known as CTCs – into the peripheral blood. CTCs are associated with 90% of cancer-related deaths. They are therefore considered prognostic biomarkers in tumor metastasis and cancer diagnostics. However, the lack of an efficient commercial test has limited this approach. An interdisciplinary team – from Harvard University in the U.S. and the Second Military University and Tongji University in China – has designed and obtained a method to detect these CTCs from the buffer or peripheral blood.
Engineering magnetic isolation and fluorescent-based detection for these cells, the team used an anti-EpCAM antibody-modified, fluorescent magnetic nanobeads system (iFMNS) – in a non-destructive manner – with efficiency in the range of 70-95%.
Notably, these small biocompatible nanobeads were found to have no impact on the viability and propagation of the CTCs. This is majorly important for testing the isolated cells using cellular and molecular analysis techniques. Because this technique is non-destructive, the isolated cells could be directly used for culture, reverse transcription-polymerase chain reaction (RT-PCR) testing, and immunocytochemistry (ICC) identification.
A monoclonal anti-EpCAM (epithelial cell adhesion molecule, or CD326) antibody is conjugated with the nanobeads via streptavidin-biotin bridges. It binds to the EpCAM molecules present on the surface of the CTCs. The obtained anti-EpCAM fluorescent magnetic nanoprobes enable direct visualization on the surface of captured CTCs without additional labeling. The ultrabright fluorescence (encapsulated with many quantum dots) improves the fluorescent immunoassay sensitivity compared with single quantum dots immunoprobe.
From the magnetic hysteresis loop characterization, the researchers show that these nanobeads have an excellent superparamagnetic property at room temperature with a tunable magnetic saturation value (41.6 emu/g and 6.04 emu/g) by adjusting the concentration of magnetic nanoparticles added.
In this approach, the researchers prepared the fluorescent magnetic nanobeads by an oil-in-water emulsion-evaporation technique, using poly(styrene-co-maleic anhydride) (PSMA) as the matrix material for nanobeads. They precipitated the polymer with the entrapped quantum dots and magnetic nanoparticles inside polymer nanobeads – forming polymer/nanoparticle hybrid nanobeads.
These nanobeads are 114 nm in diameter (the average hydrodynamic size), with a polydispersity index (PDI) of 0.13, indicating good monodispersity. The researchers claim considerable colloidal stability – no aggregation or precipitation throughout the process.
In this method, the researchers incubated the nanobeads with the cells for 10 minutes. They separated the cell suspension by applying a magnetic column separator and counted the captured cells on a hemocytometer under a bright light microscope.
“Due to the mild reaction process and the polymer matrix isolation between QDs and MNPs, QDs encapsulated in the polymer nanospheres still possess high fluorescent intensity.”
The researchers further validated this technique on peripheral blood samples from a lung cancer patient, with high efficiency – successfully supporting the potential clinical application of this method. They observed that the CTCs were specifically recognized in the blood samples from WBCs, confirming that the as-constructed system could efficiently isolate and identify CTCs to mimic clinical samples.
They also show that the magnetic separation using iFMNS does not induce tumor cell apoptosis (programmed cell death). The iFMNS captured tumor cells proliferated without a significant change in behavior and morphology compared with the control samples of SGC-7901 cell lines.
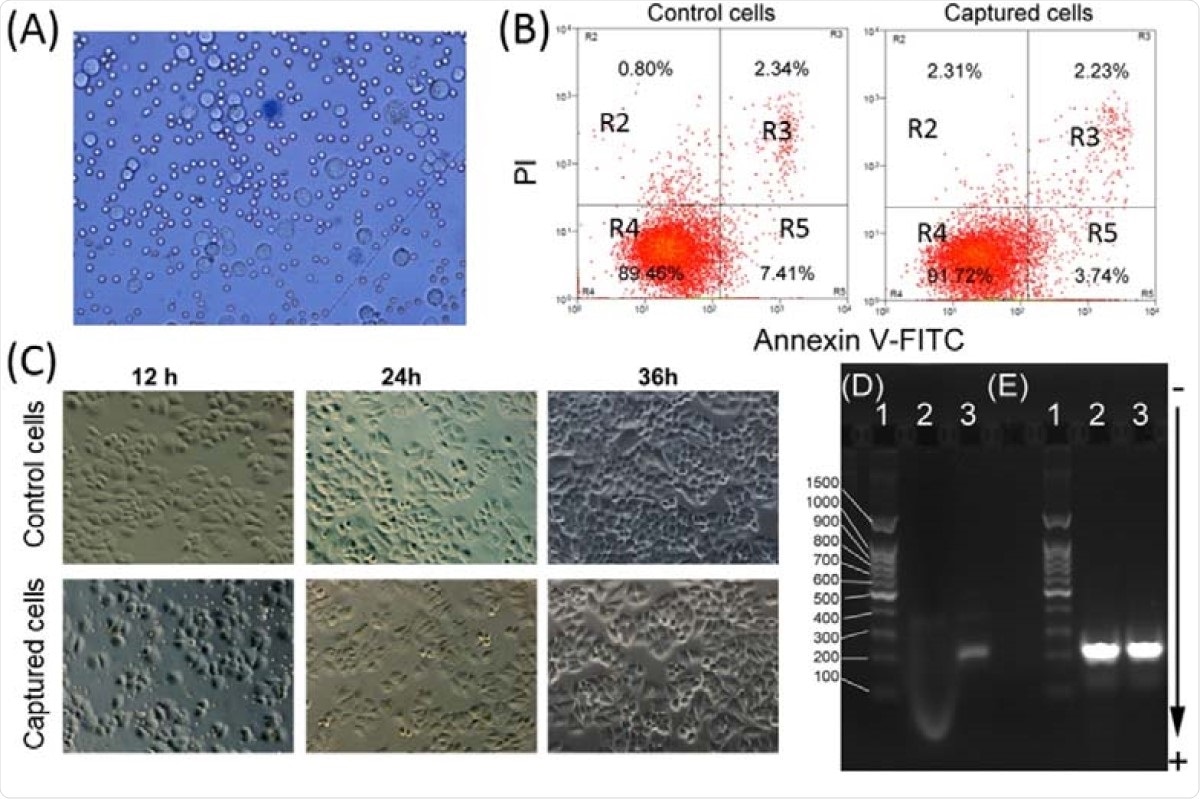
Viability and PCR analyses of the captured tumor cells. (A) Microscopic image of captured cells dyed by trypan blue. (B) Apoptosis analysis of the control of tumor cells and captured tumor cells by flow cytometry. At least 10,000 cells were measured per sample. The proportion (%) of cell number is shown in each quadrant. The proportion of viable cells was shown in the R4 quadrant (FITC-/PI-), early apoptotic cells shown in the R5 quadrant (FITC+/PI-), late apoptotic/necrotic cells shown in the R3 quadrant (FITC+/PI+). (C) Microscopic images of the control and captured tumor cells were plated and cultured for 12, 24, and 48 h. Agarose gel electrophoresis of products from RT-PCR amplification of EGFR mutation (D) and GAPDH (E). (Lane 1: DNA ladder, Lane 2: A549 cells captured with iFMNs, Lane 3: HCC827 cells captured with iFMNs).
Cadmium-containing quantum dots are limited in biological labeling – mainly due to their toxicity for cell growth. In this study, the observed impact on cell growth and apoptosis may be attributed to the thick polymer shell encapsulation around the quantum dots that prevented any cadmium ions leakage.
Moreover, the researchers also investigated the captured tumor cells for nucleic acid molecular analysis, looking for any possible damage. They find that this approach has no influence on the RNA extraction and PCR reaction. The RT-PCR can be used to analyze the isolated cells without dissociating the iFMNS.
The developed ultra-bright iFMNS are potential for rapid and simple CTCs isolation and can be adopted for multiplexed labeling to improve CTCs testing and analysis using QDs with different colors and fluorescence signals.”
This paper presents a simple, biocompatible and cost-effective approach for the simultaneous capturing, isolation, and detection of CTCs using an immuno-fluorescent magnetic nanobead system (iFMNS) coated with a monoclonal anti-EpCAM antibody. With efficiency in the range of 70-95% of the tested cells, the viability of 95% of the captured cells, and the quick results turnaround time, this method could be advantageous over the current techniques available.
In summary, the team has demonstrated an ultra-bright fluorescent magnetic nanobeads system that was successfully constructed for simultaneously efficient capture and sensitive detection of CTCs in whole blood by combining quantum dots, magnetic nanoparticles, and anti-EpCAM antibody.
The ability to perform subsequent molecular biological analysis, which was crucial for further clinical diagnosis and research, is a highlight of this approach.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Pengfei Zhang, Mohamed S. Draz, Anwen Xiong, Wannian Yan, Huanxing Han, Wansheng Chen. Immunoengineered Nanobead System for the Isolation and Detection of Circulating Tumor Cells. bioRxiv 2021.01.18.427201; doi: https://doi.org/10.1101/2021.01.18.427201, https://www.biorxiv.org/content/10.1101/2021.01.18.427201v1.
- Peer reviewed and published scientific report.
Zhang, Pengfei, Mohamed S. Draz, Anwen Xiong, Wannian Yan, Huanxing Han, and Wansheng Chen. 2021. “Immunoengineered Magnetic-Quantum Dot Nanobead System for the Isolation and Detection of Circulating Tumor Cells.” Journal of Nanobiotechnology 19 (1). https://doi.org/10.1186/s12951-021-00860-1. https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-021-00860-1.