With the emergence of COVID-19 (coronavirus disease 2019), researchers have been closely investigating the complex interplay between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the human immune system, and COVID-19 disease severity.
Signaling proteins called cytokines cascade up to dangerous levels, leading to a 'cytokine storm' that damages the body's cells (self). High levels of intravascular neutrophil extracellular traps (NETs) or inflammatory cell remnants amplify thrombosis. Synergistically, all of these results in macrovascular and microvascular thrombosis during a SARS-CoV-2 infection.
Although the endothelial cell activation is identified as part of the COVID-19 thrombo-inflammatory storm, this activation's upstream mediators are unknown. In a recent medRxiv* preprint publication, researchers report for the first time that the serum from COVID-19 patients - containing circulating antiphospholipid antibodies - activates endothelial cells to express surface adhesion molecules (namely E-selectin, VCAM-1, and ICAM-1). These surface adhesion molecules are known for their role in thrombosis.
The interdisciplinary team led by Dr. Jason S. Knight and Dr. Yogendra Kanthi found that for at least a subset of serum samples, this activation could be completely mitigated by depleting the samples of Immunoglobulin G (IgG).
"Prothrombotic aPL Abs associated strongly with these phenotypes suggesting a new mechanism by which these autoantibodies may perpetuate thrombo-inflammation in COVID-19."
Scientists studying COVID-19 are increasingly highlighting the role of autoantibodies, which engage cell surfaces, activate endothelial cells, platelets, and neutrophils, tipping the blood-endothelium interface toward thrombosis. Previously this team had found that COVID-19 IgG fractions enriched for antiphospholipid antibodies (aPL Abs) potently activate neutrophils in vitro while also potentiating thrombosis when injected into mice.
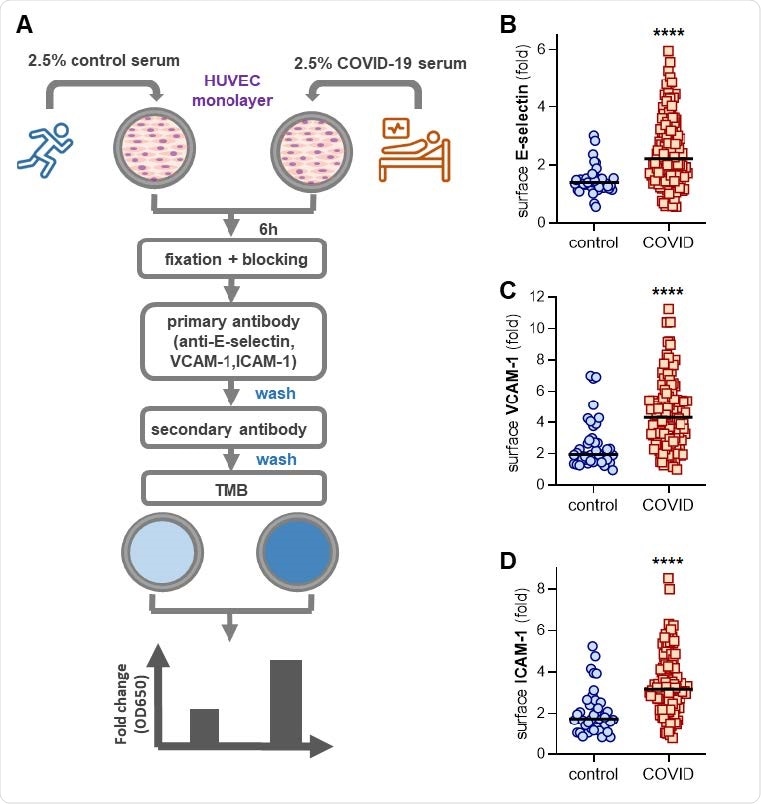
Activation of human umbilical vein endothelial cells (HUVEC) by control or COVID-19 serum. (A) Schematic workflow for in-cell ELISA. HUVEC were cultured for 6 hours with serum from either healthy controls (collected pre-pandemic) or patients hospitalized with COVID-19. Cells were then fixed and surface expression of E-selectin (B), VCAM-1 (C), or ICAM-1 (D) was quantified. Median values are indicated by horizontal lines. Groups were analyzed by Mann-Whitney test; ****p<0.0001.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
In the present study, the researchers demonstrate their hypothesis that circulating factors such as neutrophil extracellular trap (NET) remnants, D-dimer, or C-reactive protein might predict the COVID-19 serum samples (n=118) that most robustly activated cultured endothelial cells.
As expected, they found correlations between the serum NET remnants (cell-free DNA, myeloperoxidase-DNA complexes, citrullinated histone H3) and upregulation of surface E-selectin, VCAM-1, and ICAM-1 on endothelial cells. However, interestingly they also found that the presence of circulating antiphospholipid antibodies (specifically anticardiolipin IgG and IgM and anti-phosphatidylserine/prothrombin (anti-PS/PT) IgG and IgM), as markers of COVID-19 serum with strong endothelial cell activating-potential.
The study cohort included 118 patients hospitalized with moderate-to-severe COVID-19 at an academic hospital. The serum from these COVID-19 patients was diluted in culture media and then added to early-passage human umbilical vein endothelial cells (HUVEC). Any expression of surface activation markers was determined after 6 hours by a custom in-cell ELISA. For control, the serum samples were collected from 40 healthy individuals.
The researchers found that the COVID-19 samples triggered an activated endothelial cell phenotype, evidenced by increased surface expression of the leukocyte adhesion molecules E-selectin, VCAM-1, and ICAM-1.
Looking for upstream mediators that activate the endothelial cells, the researchers measured NETs in serum and the general markers of thrombo-inflammation: C-reactive protein, D-dimer, and neutrophil calprotectin. Their results demonstrate modest correlations between NETs, thrombo-inflammation, and the ability of COVID-19 serum to activate endothelial cells.
In this study, the researchers detected a strong correlation between all four antibodies (IgG and IgM isotypes of two types of aPL Abs (anticardiolipin and anti-PS/PT) and the three markers of endothelial cell activation (E-selectin, VCAM-1, and ICAM-1), indicating their role in the activation of the endothelial cells.
In summary, these data indicate that COVID-19 serum contains factors capable of activating the endothelial cells. The researchers cite and discuss the many recent reports of autoantibodies in COVID-19 patients and the evident association with the disease severity.
"Taken together, these findings suggest a pathological role for autoantibodies in COVID-19 with diverse impacts on immune function, thrombosis, and likely clinical outcomes."
One of the potential clinical implications of the study proposed by the researchers is to consider whether patients with moderate-to-severe COVID-19 should be screened for aPL Abs to evaluate their risk of thrombosis and progression to respiratory failure. Subsequently, such patients may benefit from alternate treatment strategies.
Importantly, this results from this study also suggest other potential therapies, including monoclonal antibody-mediated depletion of plasmablasts, the specific inhibition of B cells reactive against phospholipids and phospholipid-binding proteins, endothelial cell-stabilizing therapies, and manipulation of pathogenic-antibody Fc glycosylation or afucosylation - all are potentially worthy of future research, the researchers write. This study calls for further investigations to understand how aPL Ab-associated IgG fractions activate the endothelial cells.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Endothelial cell-activating antibodies in COVID-19 Hui Shi, Yu Zuo, Alex A. Gandhi, Gautam Sule, Srilakshmi Yalavarthi, Kelsey Gockman, Jacqueline A. Madison, Jintao Wang, Melanie Zuo, Yue Shi, Jason S. Knight, Yogendra Kanthi, medRxiv, 2021.01.18.21250041; doi: https://doi.org/10.1101/2021.01.18.21250041, https://www.medrxiv.org/content/10.1101/2021.01.18.21250041v1
- Peer reviewed and published scientific report.
Shi, Hui, Yu Zuo, Sherwin Navaz, Alyssa Harbaugh, Claire K. Hoy, Alex A. Gandhi, Gautam Sule, et al. 2022. “Endothelial Cell‐Activating Antibodies in COVID‐19.” Arthritis & Rheumatology, February. https://doi.org/10.1002/art.42094. https://onlinelibrary.wiley.com/doi/10.1002/art.42094.