The ongoing pandemic of coronavirus disease 2019 (COVID-19) — caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection — has led to an unparalleled focus on developing preventive and therapeutic measures. Within a year of the pandemic’s onset, the world saw the approval of the first COVID-19 vaccines for emergency use. Scores more are being developed or are in late-phase clinical trials. A new paper published in the journal Advanced Drug Delivery Reviews sums up what is known about these vaccines so far.
Many national and international research collaborations and drives have also come up as a result of the urgent need for vaccines to arrest or at least contain the spread of the virus. These are aimed at funding and coordinating various phases of vaccine development, as well as codifying and streamlining manufacturing processes and global distribution of the final vaccine. The money and efforts that have gone into such programs have reduced the standard timeline for vaccine production from 10-15 years to 12-18 months.
Vaccine concerns
The first concern for COVID-19 vaccines is safety, which is being evaluated in humans since reliable animal models are unavailable at present. Of the vaccines which have been tested so far, most seem to have few adverse events.
Efficacy is another important criterion, with the US Food and Drug Administration (FDA) recommending that vaccines have at least 50% efficacy relative to placebo. This means a reduction in the number of cases, reduction in disease severity, or reduction in the number of infections.
No study as of now has examined the interaction between different COVID-19 vaccines. The currently authorized Pfizer and Moderna vaccines are mRNA-based vaccines. Both have claimed 95% efficacy, but have different storage temperatures, dosages and dosing intervals between the priming and booster doses.
Vaccine targets
The virus is an enveloped, single-stranded, and positive-sense RNA virus. Part of the betacoronavirus family, it spreads via respiratory droplets. The infection spreads more rapidly when people are crowded indoors, in poorly ventilated rooms, and if the exposure is prolonged.
The virus has 15 non-structural proteins and 12 structural and accessory proteins. Of these, the primary vaccine targets include the spike (S), the membrane (M) and the envelope (E) antigens, on the surface of the virus, and the nucleocapsid (N) associated with the viral RNA inside the virus.
The accessory proteins may modulate the virulence and the host immune response, for instance by their effect on cytokine release and on pathways mediated by Type I interferon. Important among these are ORF3a and ORF7a, expressed on the membrane of infected cells.
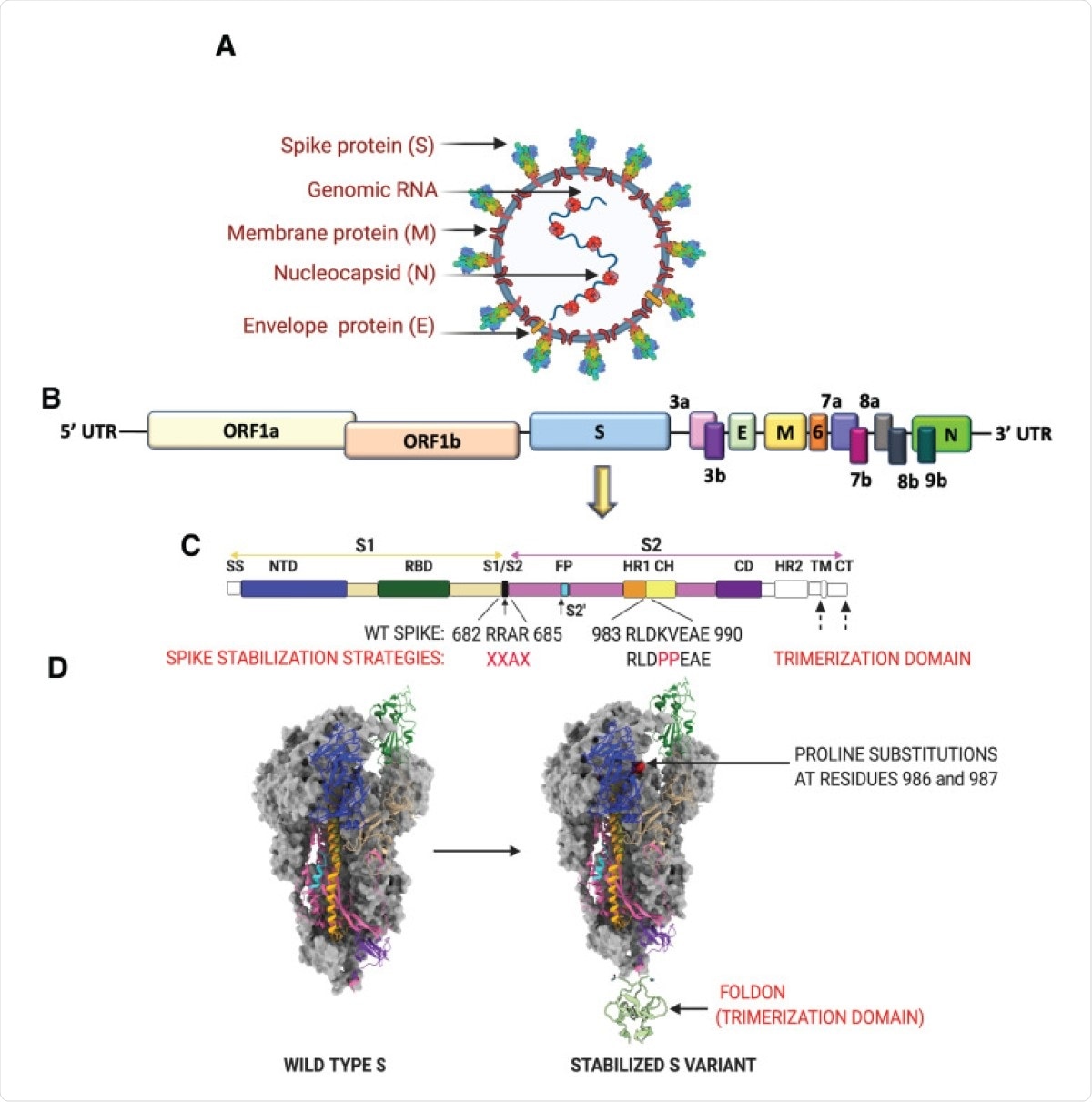
SARS-CoV-2 virion, genome and strategies for stabilizing the spike protein. (A) SARS-CoV-2 virion with structural proteins (spike (S), membrane (M), nucleocapsid (N) and envelope (E)) and genome depicted. (B) Organization of the SARS-CoV-2 genome. (C) SARS-CoV-2 S protein organization. The S1 subunit (tan) consists of a 5′ signal sequence (SS) followed by the N-terminal domain (NTD) and the receptor binding domain (RBD). Arrows denote the two protease cleavage sites: the polybasic furin site between S1/ S2 and the S2′ site. Cleavage at these two sites in the S protein exposes the hydrophobic fusion peptide (FP) and triggers the fusion process. The other domains of the S2 subunit are the heptad repeat 1 (HR1), CH-central helix, CD-connector domain, heptad repeat 2 (HR2), transmembrane domain (TM) and cytoplasmic tail (CT). Domains that have no corresponding residues in the cryo-EM structures shown in (D) are colored in white. Multiple protein engineering strategies have been adopted to stabilize the pre-fusion conformation. Most of the full-length vaccine candidates have adopted one or all of the following strategies: 1) Introduction of two stabilizing proline mutations at residues 986 and 987 in the loop between HR1 and CH, 2) Removal of the polybasic cleavage site between S1 and S2, and 3) Stabilizing the trimeric spike by addition of a trimerization motif to maintain integrity of conformational epitopes. (D) Prefusion structure of the SARS-CoV-2 spike protein determined by cryo-EM (PDB ID: 6VSB). The spike is a homotrimeric protein. Two of the monomers are colored in gray, whereas the various structural domains have been mapped on one of the monomers in the identical color as shown in (C).
The spike protein is the major target of neutralizing antibodies, which are most closely associated with protective responses against viral infection in humans. Composed of two subunits, it mediates both virus-host attachment via its receptor-binding domain (RBD) and virus entry into the host cell.
Stabilized spike protein
The titers of anti-RBD and anti-spike immunoglobulin G (IgG) closely follow neutralizing antibody titers in vitro. Many vaccines use stabilized prefusion spike protein antigen, because of its ability to potently induce a neutralizing response. One such stabilized spike antigen contains two proline substitutions (S2P), which prevent conformational rearrangements in all betacoronavirus spike proteins.
Severe COVID-19 is associated with high pro-inflammatory IgGs, that provoke FcγR-mediated hyperinflammatory reactions, causing antibody-dependent enhancement (ADE) or vaccine-associated enhanced respiratory disease (VAERD). This is not considered to be a potential adverse reaction of COVID-19 vaccines.
The N protein is also very immunogenic and is targeted by both antibody and T cell-mediated responses. Though anti-N antibodies are likely not protective, and high anti-N antibody titers indicate a poor outcome, T cell-mediated immunity against the N protein may help clear the virus.
Vaccine platforms
The types of vaccine platforms represented among COVID-19 vaccine candidates include both conventional and novel platforms. The former comprises the inactivated virus (IV), its recombinant protein-based subunits (PS), live attenuated virus (LAV) and virus-like particles (VLPs).
Newer platforms include those based on nucleic acid, namely, DNA and mRNA encoding the gene of interest (GOI), and those based on viral vectors, both non-replicating viral vector (NRVV) and replicating viral vector (RVV).
IVs allow exposure to all the viral proteins. The chief danger is that of eliciting non-protective antibodies following antigen breakdown, and of a deleterious T cell response. In the current pandemic, these can be produced only at biosafety level-2 (BSL-2) laboratories, with highly skilled workers.
LAVs are meant to reflect the natural exposure of the host to the virus, producing robust long-term immunity. Mucosal immunity may be generated following intranasal administration. Reversion to the wildtype requires very stringent quality checks and viral characterization; they are contraindicated in very old or immunocompromised individuals; and they must be stored in cold temperatures.
Viral vectors are platforms that use either attenuated or non-replicating viruses that encode the immunogen (in this case, the spike protein) in their nucleic acid, thus acting like LAVs. Pre-existing immunity against a human-infecting viral vector may inadvertently prevent immune responses against the vaccine antigen as well, making the vaccine ineffective. For this reason, chimpanzee adenoviruses are used.
No VV-based vaccine has ever been licensed so far, but early data on the safety and immunogenicity of Ebola and chikungunya vaccines show good results.
Four of ten such COVID-19 vaccine candidates are NRVV-based, using vectors that cannot replicate and just insert the spike-encoding region into the host cell. RVV-based COVID-19 vaccines can replicate following their injection, resulting in higher immunity levels and lower doses.
Nucleic acid, either DNA or RNA, can be delivered into the host cell. The nucleic acid that encodes the viral antigenic proteins can be delivered by plasmids into the host cell. They can be rapidly upscaled or downscaled.
DNA vaccines are based on a bacterial plasmid that contains a clone of a GOI and a strong upstream promoter region, which is inserted into the host organism. The plasmid must then pass into the nucleus and be transcribed, and then the RNA will be translated in the cytoplasm, generating the immunogenic viral protein target.
RNA-based platforms encode the stabilized prefusion S protein, and are either self-amplifying or non-replicating. The spike-encoding RNA vaccines are enclosed in lipid nanoparticles (LNPs) and can be directly translated into the required viral antigen. They also lead to effective antigen presentation and activate the B cells as expected. Their stability is a potential obstacle, as is the paucity of safety data in humans on a large scale.
PSs are both safe and effective, and many strategies have been evolved to boost the response. One is adjuvanting the vaccine; another is using multimeric antigens, or conjugating the antigen to carrier nanoparticles. They are more expensive and cumbersome to produce, but the technology has been widely used for many licensed vaccines.
VLPs are a special type of recombinant viral proteins, and comprise viral proteins that assemble themselves into geometrical structures that mimic the viral particles themselves. Typically very immunogenic, they are able to induce robust humoral and cell-mediated innate and adaptive immunity.
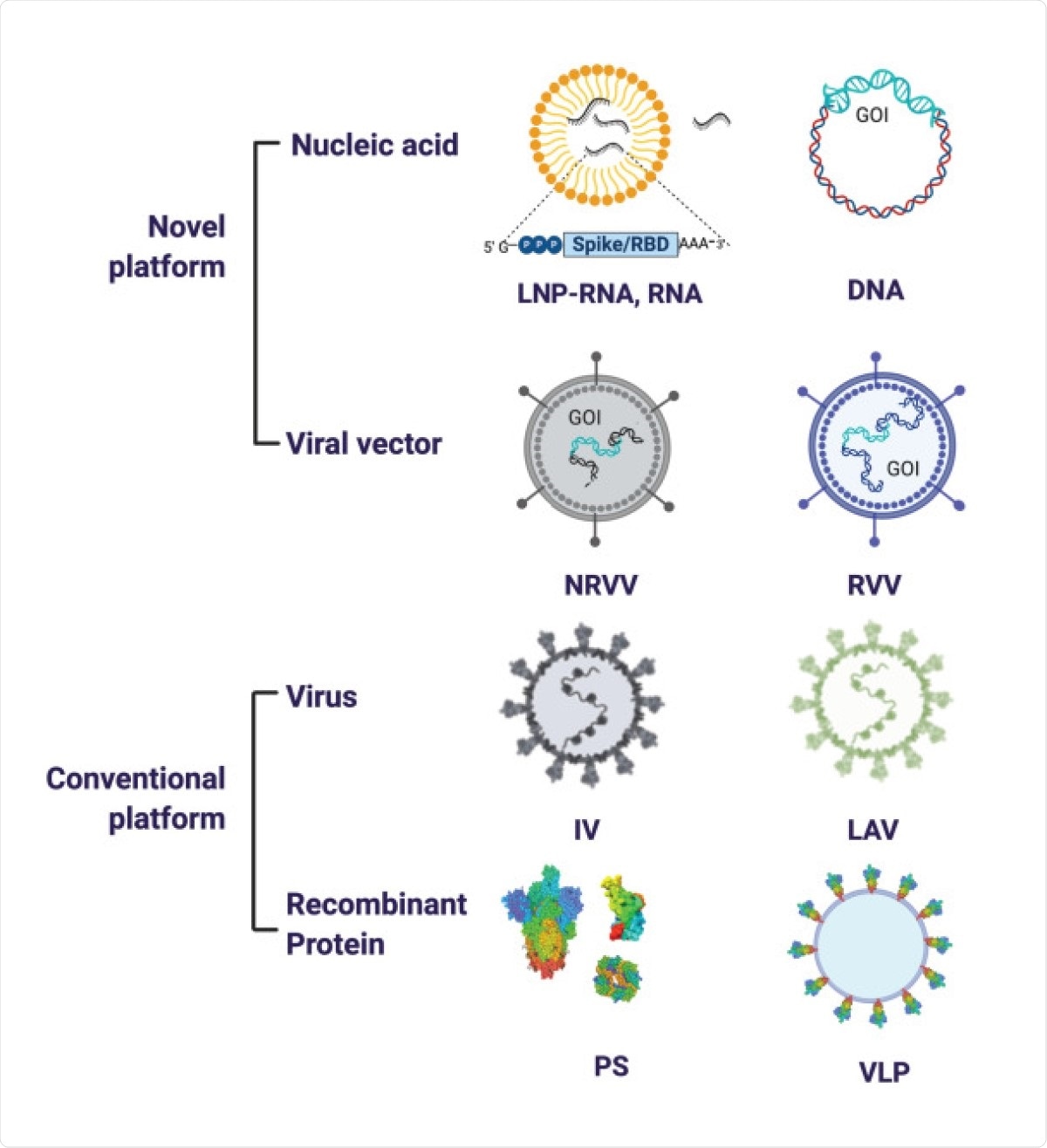
SARS-CoV-2 vaccine platforms. The major vaccine platforms that are being used in current SARS-CoV-2 vaccine candidates. A combination of conventional and novel vaccine platforms are being tested. Conventional vaccine platforms that have been licensed for human use are the inactivated virus (IV), live attenuated virus (LAV), and recombinant protein-based (Protein Subunit (PS)). Novel vaccine platforms include nucleic acid based (DNA and RNA encoding the gene of interest (GOI)) and viral vector-based (non-replicating viral vector (NRVV) and replicating viral vector (RVV).
COVID-19 vaccines in phase III trials
A sampling of vaccines using these platforms, that have passed early safety and efficacy tests, include three IVs, namely, BBIBP-CorV vaccine, from the Beijing Institute of Biological Products/Sinopharm, an unnamed one from the Wuhan Institute of Biological Products/Sinopharm, and the Coronavac IV vaccine from Sinovac.
VV-based vaccines include the adenovirus-based Ad5-nCoV and the Ad26.COV2.S with the transmembrane domain of the spike, the cytoplasmic tail and the S2P mutation; AZD1222 from Oxford University and AstraZeneca, which has been authorized for emergency supply for active immunization of adults; and the Russian Sputnik V. The latter was controversially released for clinical use without phase III trials and is said to have an efficacy of over 90%. Simultaneously, a phase III trial is ongoing.
The Moderna mRNA-1273, and the Pfizer/BioNTech BNT162b2 are both mRNA vaccines, the latter being an LNP-encapsulated nucleoside-modified mRNA. Both have been authorized for emergency use for active immunization to prevent COVID-19 in those aged 18, and 16 and above, respectively.
Protein subunit vaccines include NVX-CoV2373.
What are the implications?
Interestingly, all these vaccines show a dose-dependent increase in the immunogenicity and adverse reactions, indicating the need to balance these two. The optimal prime-boost interval appears to be, as usual, 21-28 days. All were associated with high seroconversion rates and high titers of neutralizing antibodies.
At the same time, high-risk populations pose their own challenges, and phase III studies have not addressed these. The safety of these vaccines in such groups thus remains unknown. The durability of immunity is also unknown and will not be an important consideration at this stage, when the priority is to arrest the pandemic.
More research will be required to understand what determines immunity and antiviral protective responses, beyond the undoubted role of anti-spike neutralizing antibodies. Broad immunity against betacoronaviruses is a desirable goal in view of the certainty of viral adaptation, to allow for protection against the inevitable emergence of different strains and variants.
And finally, vaccine uptake must be high, over 67% at least, to achieve population or ‘herd immunity’. This will be difficult with the current high levels of vaccine hesitancy, even among healthcare workers.
We need to dramatically improve education about how vaccines work and how they have been used to control infectious diseases over the past ~100 years.”