As the coronavirus disease 2019 (COVID-19) pandemic passes the grim milestone of over one hundred million reported cases, the rollout of some vaccines offers a distinct gleam of hope that some degree of control could be achieved within a year. Now, a new preprint research paper describes a chimpanzee adenovirus-vectored vaccine that, when administered intranasally in rhesus macaques, led to a robust immune response and demonstrated protection against infection by the virus that causes COVID-19, namely, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The preprint is published on the bioRxiv* server.
In a previous study, the vaccine candidate demonstrated protection against infection of the upper and lower respiratory tract in mice expressing the viral receptor, the human angiotensin-converting enzyme 2 (ACE2). The current study extended these findings to show its efficacy in non-human primates.
The SARS-CoV-2 spike antigen
The virus enters the host cell via its spike antigen's engagement with the cell surface ACE2 receptor. The spike (S) protein is thus a critical vaccine target and the focus of the development of therapeutic antibodies. The S protein is cleaved in multiple steps, first at the S1/S2 interface. The S1 subunit is responsible for receptor binding at the receptor-binding domain (RBD), and then within the S2 domain to yield the S2' protein that mediates viral-cell membrane fusion.
Neutralizing antibodies recognize the prefusion S protein, which has the RBD in its 'up' conformation. The vaccine candidates under development include the DNA/RNA vaccines, lipid nanoparticle (LNP)-encapsulated mRNA, inactivated viral vaccines, protein subunit vaccines, and viral vectored-vaccines.
Failure to prevent viral spread
Most currently available vaccines, and those undergoing phase III trials, require two doses and are intramuscularly administered. These conditions not only require a more extended period of time for protective immunity to set in but do not confer local or mucosal immunity, allowing viral shedding to continue.
Therefore, the vaccinated individuals can still transfer the infectious virus to others even though they are protected against the infection themselves. (The Moderna vaccine has been shown to reduce viral shedding from the nose in experimental animals, but human data is lacking.)
Experiments with intramuscular (IM) vaccines in non-human primates (NHP) show that despite the protection they offer against COVID-19 pneumonia, the same cannot be said of upper airway infection and, therefore, against viral transmission.
Different vaccine antigens
The researchers described the use of chimpanzee Adenovirus (simian Ad-36)-based SARS-CoV-2 vaccine (ChAd-SARS-CoV-2-S) that expresses the S protein. Earlier, when given intranasally to mice, a single dose elicited protective antibodies and cell-mediated immunity against the spike protein and prevented infection of both the upper and lower airways.
The spike protein used in this vaccine candidate (ChAd-SARS-CoV-2-S) differs from the other chimpanzee Ad-23-based vaccine, ChAdOx1 nCoV-19, in phase III trials, in having backbone deletions that upregulate spike expression and stabilizing proline mutations to keep the protein in its prefusion form.
The current study used one intranasal dose of the vaccine in rhesus macaques (Macaca mulatta), followed by a SARS-CoV-2 challenge by both intranasal and intrabronchial routes.
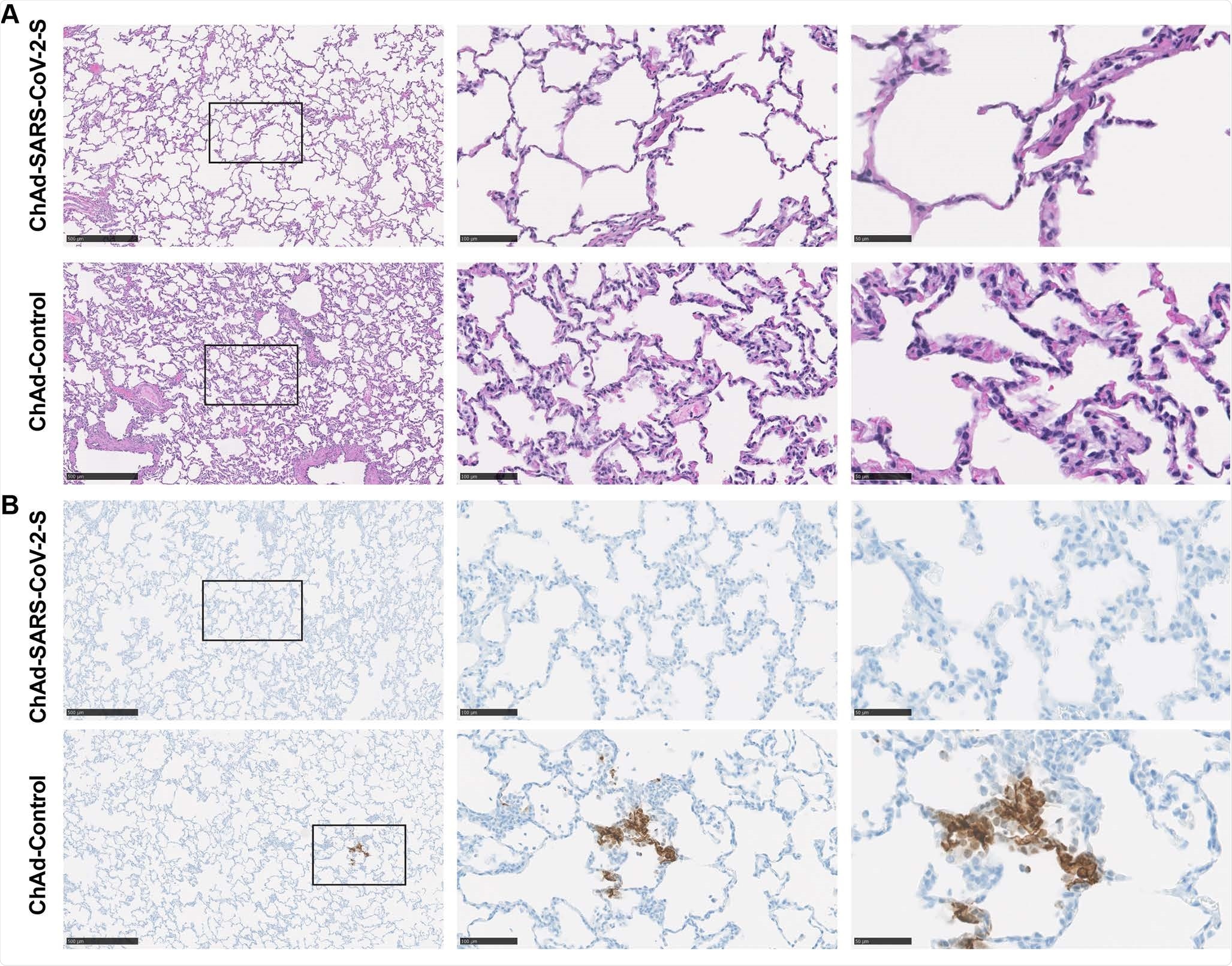
Pathological analysis of lungs of vaccinated RMs. RMs were immunized with ChAd-control and ChAd-SARS-CoV-2-S and challenged. Lungs were harvested at 7 dpi. A. Sections were stained with hematoxylin and eosin and imaged. Each image is representative of a group of 6 RMs. B. SARS-CoV-2 antigen was detected in lung sections from RMs for conditions described in (A). Images show low- (left; scale bars, 500 μm), medium- (middle; scale bars, 100 μm), and high-power magnification (right; scale bars, 50 μm). Representative images from n = 6 RMs per group.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Immunogenic vaccine
They found anti-S, anti-RBD, and neutralizing antibodies. T cell responses were also present.
Protection against local infection
In nasal swabs, viral RNA loads were lowered in immunized animals, and only one had a detectable infectious virus on day one post-challenge, compared to four of six controls. At later time points, no infectious virus was isolated from any nasal swab.
This suggests that local infection is prevented, with lower viral RNA levels and more rapid viral clearance.
Protection against lung infection
The researchers also measured the infection level in bronchoalveolar lavage fluid (BALF) on day 1 and 3 post-challenge and lung tissues (day 7), from exposed animals. One of six samples on day 1 were positive for infectious virus, vs all control samples. The viral titer was also less by three orders of magnitude.
On day 3, only one sample from a control animal was positive for infectious virus. Viral RNA was increased a hundred-fold and fifty-fold, on day 1 and 3, respectively, in control BALF samples relative to immunized animals, supporting viral clearance.
The lung tissues in controls also showed a much higher level of viral RNA in immunized animals vs. controls on day 7. In fact, the higher the neutralizing antibody titer, the lower the viral RNA in BALF. This correlation was more sensitive than that of anti-S IgG, indicating that the titer of neutralizing antibody may be proportional to the level of protection offered by this vaccine.
Viral antigen staining in the lungs of immunized animals was absent, vs. four of six control animals.
What are the implications?
Both at the entry site and in distant tissues, a single dose of the ChAd-SARS-CoV-2-S vaccine could significantly reduce the incidence of disease and viral spread. Of course, there was a low incidence of severe lung pathology, or disease, in both controls and immunized animals, despite the use of high doses of infection and introduction of the infectious virus by both intranasal and intrabronchial routes. This limits any conclusion as to the protective efficacy of this vaccine against SARS-CoV-2-induced disease.
"As this single intranasal dose vaccine confers protection against SARS-CoV-2 in non-human primates, it is a promising candidate for limiting SARS-CoV-2 infection and transmission in humans." Further research could focus on ways to increase the measure of protection, including the potential use of a homologous or heterologous booster dose with the same or another adenovirus vectored-vaccine.
The absence of vaccine-dependent enhancement of disease is another encouraging sign. However, further research is required to compare the immunity achieved with intranasal and IM administration of this vaccine, as well as the timescale of immunity over time following intranasal vaccination with ChAd-SARS-CoV-2-S.
Source

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources