The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen, has dominated human life all over the world for almost a year. Since the identification of this illness in December 2019, it has taken over 2.2 million lives. Meanwhile, immense economic stress has resulted from the severe restrictions imposed upon human mobility and physical interactions outside one’s own family or household to contain the spread of the virus.
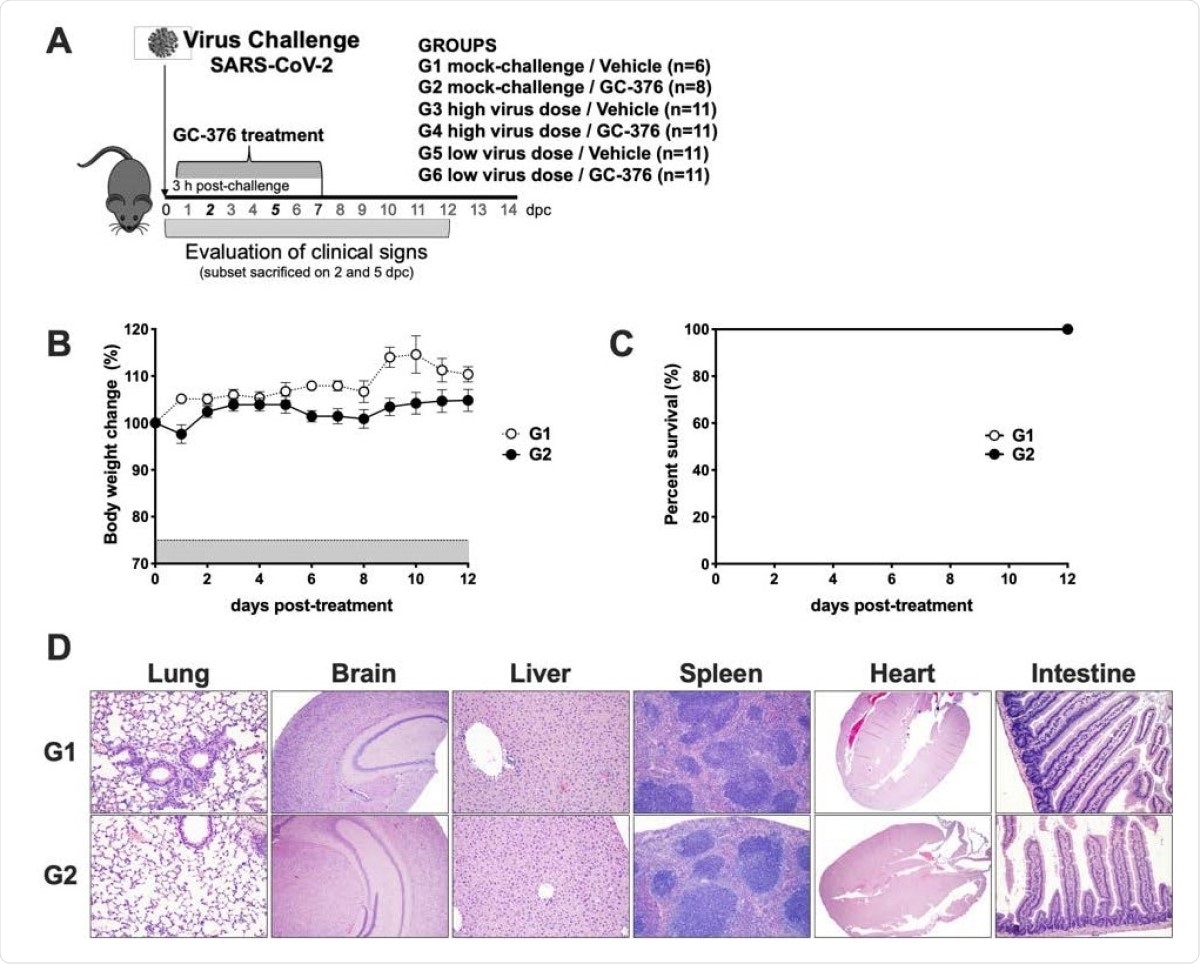
Schematic representation of the experimental design and evaluation of the safety of GC-376 in K18-hACE2 mice. (A) K18-hACE2 were either mock challenged (G1 and G2) or challenged with SARS-CoV-2 at either a high virus dose (1x10ˆ5 TCID50/mouse; G3 and G4) or a low virus dose (1x10ˆ3 TCID50/mouse; G5 and G6). I.P. treatment with vehicle (G1, G3, and G5) or GC-376 (G2, G4, and G6, 40mg/kg day). Subsets of mice were humanely euthanized at 2 and 5 dpc and different tissues were collected. Mice (n=8) received 40mg/kg of GC-376 split in two doses per day for 7 days and (B) weight changes and (C) survival were monitored for 12 days. (D) Mice were humanely euthanized at 14 dpc and lungs, brain, liver, spleen, heart and SI were collected. HE slides were generated and analyzed in comparison with the negative control group. Representative pictures were taken at 20X magnification for all tissues except brain samples that were taken at 4X.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Evaluating GC-376 in vivo
The most feasible way to contain this virus is to vaccinate the global population, and this has led to the accelerated development and release of several COVID-19 vaccines. Over the past months, many potential antivirals have also been identified as deserving further development to target several viral proteins. A recent preprint on the bioRxiv* server deals with one such molecule, the viral main protease (Mpro) inhibitor GC-376.
While in vitro studies have demonstrated the inhibition of SARS-CoV Mpro in the presence of GC376, and its potential anti-SARS-CoV-2 activity, it has not, like other Mpro inhibitors, been tested in vivo. The current paper aims to report the in vivo antiviral activity of GC-376.
Study details
The researchers used the transgenic K18-hACE2 mouse model, which comprises mice that have been modified by the introduction of the human angiotensin-converting enzyme type 2 (hACE2) receptor under the control of the keratin 18 promoter. This animal model develops the clinical disease and lethal infection following challenge with the SARS-CoV-2.
The researchers challenged the mice with two different viral doses of SARS-CoV-2. Controls were mock-challenged. Three hours after the viral challenge, the mice were treated with GC-376 or a vehicle for seven days.
The mock-challenged mice showed no ill-effects whether treated with the vehicle or GC-376. The virus-exposed GC-376-treated mice developed dose-dependent effects in terms of clinical disease.
Mice in the high-dose challenge group, untreated, either died or became so severely ill that they were euthanized. In the high-dose challenge GC-376-treated group, there was a similar outcome, but one in five mice survived. Though this difference failed to attain significance, the loss of body weight between the two groups was significantly different.
In the low-challenge untreated group, disease progression occurred albeit at a slower rate, indicating a dose-dependent effect of viral challenge. Ultimately, 60% of challenged mice survived. However, there was no clear improvement in the clinical outcome of mice in the low-challenge GC-376-treated and untreated groups, indicating the drug has only a small benefit in terms of clinical signs and outcome.
Brain viral loads lower in GC-376-treated mice
The researchers found that mice treated with GC-376 showed high levels of replication in various organs. The viral titers increased in many tissues over time, indicating systemic spread.
However, the brain samples in GC-376-treated groups showed reduced viral loads, though the variation between individuals in the low-virus challenge GC-376-treatment groups was considerable. In this group, the viral load in the brain was often undetectable, but this was not correlated with a higher survival compared to controls.
Lack of toxicity
The detailed examination of various tissues in infected mice shows that GC-376 does not produce obvious acute toxicity, but on the other hand, the drug may alleviate the extent of inflammation and virus-induced tissue damage observed following infection.
What are the implications?
The researchers note that high virus challenge led to severe disease and uniformly lethal outcome, versus delayed disease and 60% survival in the low virus challenge group. This study confirms the suitability of this transgenic mouse model as a platform to evaluate disease outcomes and host responses, even though the mechanisms of lethality may not correspond to those operating in humans.
The study also shows the safety of GC-376. The drug-treated low virus challenge groups showed only modest differences in terms of clinical signs, weight loss, and survival from exposed controls. However, further findings include the presence of lower viral loads, milder tissue lesions and reduced inflammation compared to exposed controls.
The lower viral loads in the brain following GC-376 treatment are important since neurological involvement is seen in many COVID-19 patients, and are associated with severe or critical disease in two-thirds of the cases.
The milder lesions and the apparently favorable effect of GC-376 on virus-induced inflammation could also be important, since the dysregulated immune response with multisystemic inflammation is considered a major and often fatal complication of COVID-19.
The researchers suggest that this shows that GC-376 can act as a SARS-CoV-2 antiviral in vivo. Its modest activity was expected from its moderate activity in vitro. The efficacy would need to be improved by ten- to a hundred-fold in order to present useful therapeutic activity.
This may be possible by optimizing the structure of the compound, using higher doses or by combining it with other antivirals or anti-inflammatory drugs like dexamethasone. Future studies in this direction would be useful.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources