It is well known that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of the coronavirus disease 2019 (COVID-19) pandemic, invades the human host cell through its spike protein. This protein engages with the angiotensin-converting enzyme 2 (ACE2) receptors on the human cell, enabling the virus to infiltrate and hijack its metabolic mechanisms for the purposes of viral replication.
A new nanomedicine, called “Nanotraps” – a family of nano-enabled virus-trapping particles – have been developed by a team of researchers at the University of Chicago, USA. These nanoparticles are designed to block spike protein-ACE2 interaction and trigger the macrophages to engulf and clear the virus without becoming infected.
The research team functionalized nanoparticles with either recombinant ACE2 proteins or anti-SARS-CoV-2 neutralizing antibodies and phagocytosis-specific phosphatidylserines to make these Nanotraps. These biodegradable Nanotraps effectively captured SARS-CoV-2 and completely blocked the SARS-CoV-2 infection to ACE2-expressing human cell lines and primary lung cells.
This team’s findings were recently published on the bioRxiv* preprint server.
What are "Nanotraps" and how do they work?
The interdisciplinary research team has engineered a Food and Drug Administration (FDA)-approved polylactic acid (PLA) polymeric core, a liposome shell, surface ACE2/neutralizing antibodies, and phosphatidylserine ligands to devise the Nanotrap.
The design of our Nanotraps was inspired by the ability of tumor cells to secrete PD-L1 exosomes, which bind to and suppress T-cell immune functions and thus prevent the killing and clearance of the tumor cells.”
The structures of the synthesized Nanotraps were spherical and monodispersed. Though smaller than mammalian cells, these nanoparticles were large enough to bind to several SARS-CoV-2 virions.
The researchers increased the ACE2 or neutralizing antibodies on the Nanotraps to target selective virus containment as compared to the ACE2 expressing host cells. The Nanotraps outperformed the soluble ACE2 or antibody counterparts to capture and contain SARS-CoV-2.
After containing the virus, the team aimed to clear it via macrophage-mediated phagocytosis. They used phosphatidylserine ligands on the nanoparticles. Phosphatidylserine coatings enhance the uptake of the liposomal nanoparticles by macrophages. Both the phagocytosed pseudotyped and authentic virus, are cleared in vitro.
Our in vitro neutralizing experiments demonstrated that our Nanotraps not only served as a sponge to capture and contain SARS-CoV-2 but also utilized the phagocytosis and sterilization machinery of macrophages to defend the host cells from infection.”
When tested for pseudotyped SARS-CoV-2 entry into susceptible ACE2- overexpressing HEK293T cells, lung epithelial A549 cells, and human primary lung cells, as well as authentic SARS-CoV-2 infection of Vero E6 cells, the researchers observed that these Nanotraps completely blocked the virus.
Furthermore, the researchers also demonstrated an excellent biosafety profile of the Nanotraps in vitro (cell culture) and in vivo (intratracheal administration of Nanotraps to immunocompetent mice). The researchers found no difference between the Nanotraps-treated mice and the controls.
Finally, they also demonstrated the Nanotraps inhibiting the pseudotyped SARS-CoV-2 infection in live human lungs (healthy, non-transplantable human donor lungs) using an ex vivo lung perfusion (EVLP) system.
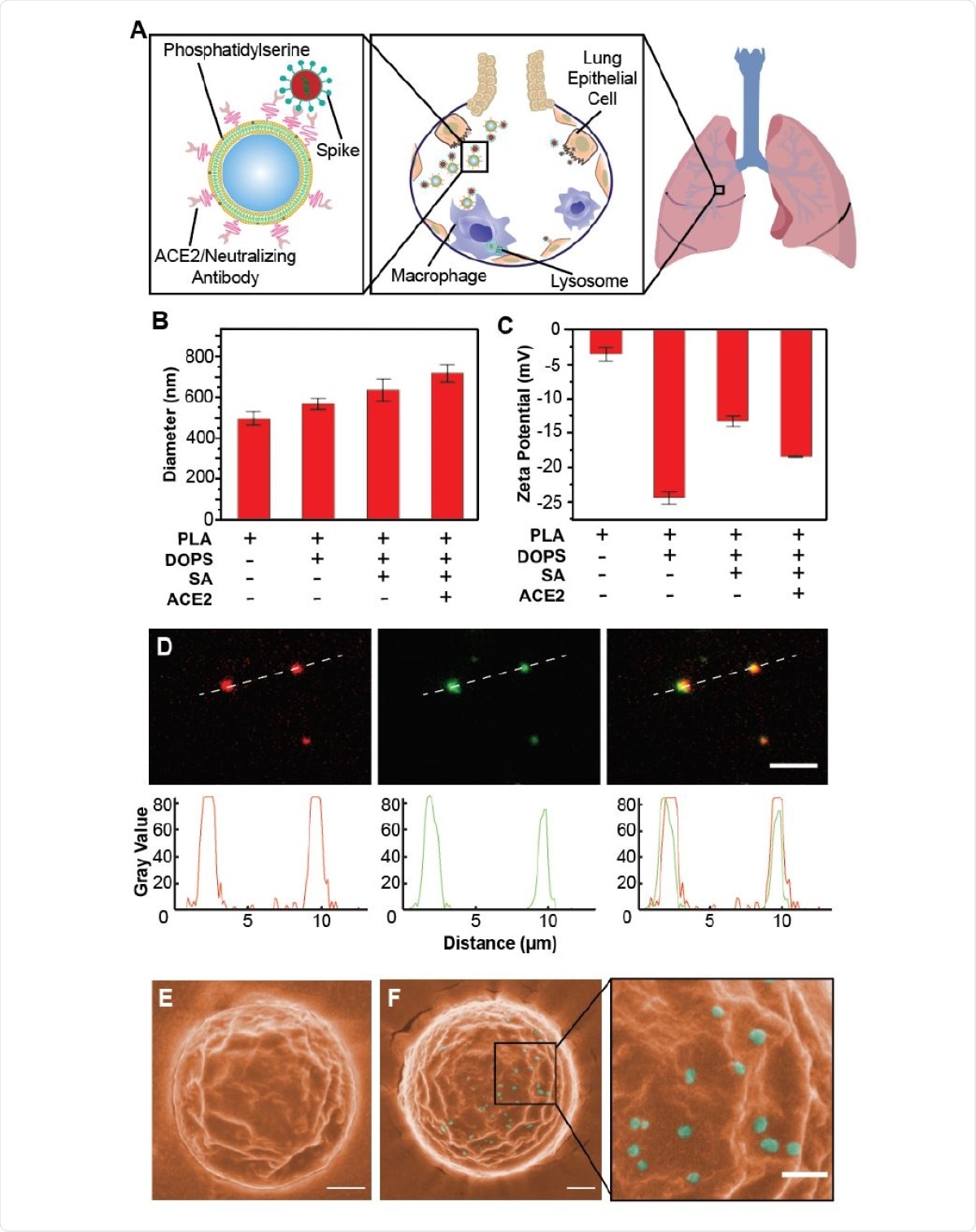
Schematic design, synthesis, and characterization of Nanotraps for SARS-CoV-2. (A) Schematic illustration showing the process of the Nanotraps with polymeric core coated with lipid-bilayer functionalized with ACE2 protein/neutralizing antibody. Following intratracheal administration, Nanotraps efficiently accumulated and trapped SARS-CoV-2 virionsin the lung tissue forming virus-Nanotrap complexes, which can be cleared by macrophages via phagocytosis, thereby blocking viral cellentry. (B-C) Dynamic light scattering (B) and Zeta-potential measurements (C) during different stages of Nanotrap preparation. (D) Fluorescent images of the prepared Nanotraps with PLA polymeric core (DiD, red) and ACE2 (anti-ACE2-AF488, green). Scale bar represents 5 µm. Dotted lines represent displayed plot profile below. (E-F) Pseudocolored SEM images of Nanotraps alone (E, orange) or with SARS-CoV-2 pseudovirus (F, cyan). To better visualize the selectivity for viral binding, larger Nanotraps were imaged. Scale bar represents 300 nm.
In summary, the researchers here present Nanotraps, a new nanomedicine, for the inhibition of SARS-CoV-2 infection.
What are the implications?
SARS-CoV-2 has so far infected over 104 million people and claimed over 2.25 million lives. While the logistics of mass vaccination are still being ironed out in most parts of the world, non-pharmaceutical interventions (NPIs) – such as social distancing, face mask mandates, social or physical distancing and regional or national lockdowns – remain the dominant mitigation strategy for curbing SARS-CoV-2 transmission and infection.
At an unprecedented pace, SARS-CoV-2 vaccine candidates are being developed to prevent infection. Some have already been approved and are being administered in some parts of the world to targeted demographics that are particularly vulnerable to severe COVID-19. But global herd immunity for a host of economic, social, and practical reasons remains a distant prospect. Finding an effective way to treat those with severe or critical disease, then, remains a global public health priority.
Despite tremendous research efforts to develop or repurpose therapeutics against the infection, however, a safe, effective and targeted medicine to treat infection is yet to be approved and rolled out. Existing therapeutic options for COVID-19 patients pose many challenges, and their effect on disease outcome is often not as expected. The researchers in this study thus offer a potential answer to this pressing public health dilemma of global concern.
Their study presents a nanomedicine that is safe, effective, biocompatible, ready for mass production, and convenient to use to effectively contain and clear SARS-CoV-2 for the prevention and treatment of COVID-19.
At a time when the global scientific community is still on the hunt for safe, effective and targeted antivirals against SARS-CoV-2 infection, the researchers’ promising initial results – both in vitro and in vivo – could well present early findings on a game-changing therapeutic candidate.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Chen, Min, Jillian Rosenberg et al. (2021) Nanotraps for the containment and clearance of SARS-CoV-2. bioRxiv 2021.02.01.428871; doi: https://doi.org/10.1101/2021.02.01.428871, https://www.biorxiv.org/content/10.1101/2021.02.01.428871v1
- Peer reviewed and published scientific report.
Chen, Min, Jillian Rosenberg, Xiaolei Cai, Andy Chao Hsuan Lee, Jiuyun Shi, Mindy Nguyen, Thirushan Wignakumar, et al. 2021. “Nanotraps for the Containment and Clearance of SARS-CoV-2.” Matter 4 (6): 2059–82. https://doi.org/10.1016/j.matt.2021.04.005. https://www.cell.com/matter/fulltext/S2590-2385(21)00166-1?.