With the emergence of new and more infectious variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the induction of population immunity by mass vaccination appears to be the only definitive way to return to a ‘normal’ way of life. While some SARS-CoV-2 vaccines are already being distributed to millions of people under emergency use authorization, issues of stability and cost require the development of others.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Challenges associated with RNA vaccines
RNA vaccines have many advantages, including the ability to rapidly manufacture them against any given target, as well as in sequence-independent processes. These contribute to their relatively inexpensive and shorter development protocols, which can be crucial in a pandemic setting. In fact, both the Pfizer and the Moderna vaccines are safe and highly effective mRNA vaccines targeting SARS-CoV-2.
Both need to be stored at extremely cold temperatures (-70°C and -20°C for the Pfizer/BioNtech and Moderna vaccines, respectively). These are challenges for mass shipping and distribution, but are necessary for many reasons, including the presence of ribonucleases in multiple cell types, that can break down vaccine RNA, despite its modification for greater ruggedness.
Secondly, mRNA is large, hydrophilic, and negatively charged, which makes its penetration into through a cell’s membrane difficult. This mandates RNA delivery through lipid nanoparticles (LNPs) that form RNA/LNP complexes less than 100 nm in diameter. These not only protect the RNA but allow it to enter the cell via endocytosis.
Freezing followed by thawing can affect the stability of both RNA and LNPs, however. Alternative lipid-based systems have therefore been researched. In this context, the current study and its findings are extremely interesting.
New platform offers thermostability
The researchers developed a thermostable nanostructured lipid carrier (NLC) that can be lyophilized for easier storage, as well as being capable of delivering RNA vaccines by intramuscular injection. In liquid form, the NLC is stable under refrigeration for a year at least. When lyophilized, the NLC/RNA complexes remain effective for eight months or more at room temperature, and 21 months or more under refrigeration.
The NLC delivery system comprises an oil core, composed of both solid and liquid fats, around which surfactant molecules are arranged with a positively charged lipid. The RNA forms an electrostatic complex with the outside of the NLC.
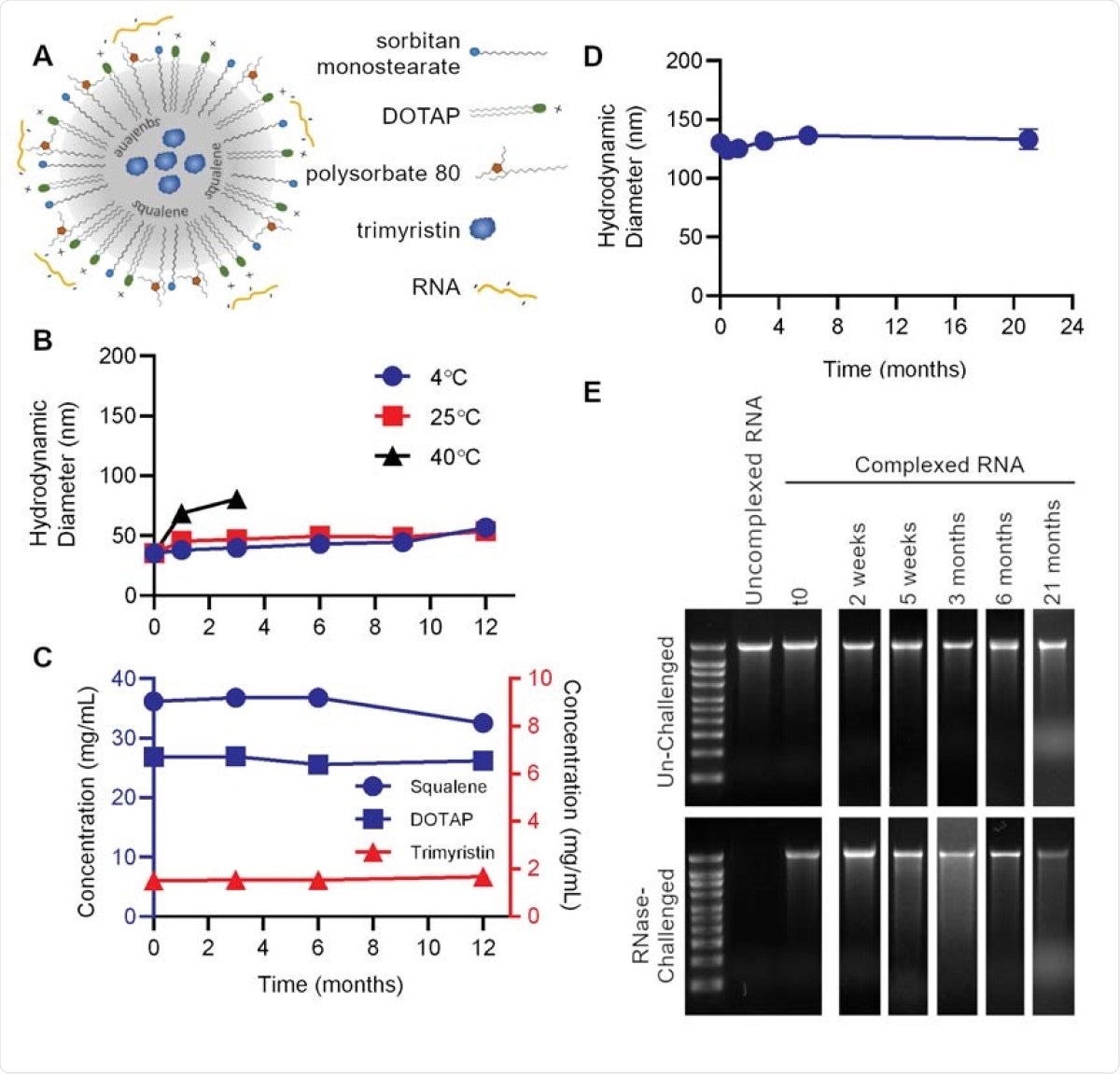
Nanostructured lipid carrier (NLC) formulation alone is stable at 4°C, allowing for stockpiling. (A) Schematic of RNA electrostatically binding to the outside of the NLC. (B) Particle size of NLC alone after storage at indicated temperatures. (C) Concentration of NLC components after long-term 4°C storage. (D) Vaccine particle size after complexing 4°C-stored 5 NLC with SEAP saRNA. (E) Protection of SEAP saRNA from RNase challenge by NLC stored at 4°C for the indicated length of time.
Future pandemic preparedness
This system remains stable with respect to the particles' size and the concentration of each component, at 4°C, while keeping the RNA intact. The manufacture of NLCs is simple, using oil-in-water emulsion technology that is already in use for vaccine preparation.
All the lipids and detergents used in this NLC formulation are commonly used in drugs approved by the US Food and Drug Administration (FDA), or have been used in many clinical trials. These complexes have also been used in non-human primates without signs of antigenicity or toxicity.
The NLC could therefore be manufactured and stored in reserve for future pandemics, ready to form complexes with the RNA construct for any given pathogen.
Demonstration of stability
Earlier, the researchers had developed an NLC/self-amplifying RNA (saRNA) vaccine against Zika virus with high neutralizing and protective efficacy. This is now demonstrated to be amenable to lyophilization by adding 10% sucrose as a stabilizer, keeping the system intact while freezing, drying and reconstituting.
In either form, the vaccine remains stable for two or more weeks under refrigeration, as well as retaining its therapeutic efficacy at unchanged levels.
The flexibility of the platform is shown by using commercial ovalbumin-encoding RNA to form complexes with the NLC. This complex remained stable against RNase enzymes. When the sucrose lyoprotectant was used at a strength of 20%, the particles increased only slightly in size after reconstitution of the lyophilized complexes.
Reporter antigen expression stability
The researchers also demonstrated the thermostability of the NLC-RNA system with a self-amplifying RNA antigen expression reporter, based on secreted alkaline phosphatase (SEAP-saRNA). The injected self-amplifying RNA can thus be easily identified in mouse serum.
When lyophilized SEAP NLC/saRNA complexes were compared with frozen (-80°C and -20C°) complexes, and with liquid NLC-RNA complexes at 4°C and 25°C, they were found to have high stability, even though stored at 4°C, 25°C, and 40°C. The researchers observed size variations of over 15% in the particles stored at -20°C, but without any impact on the expression of the protein by the NLC/saRNA complex after injection.
Reasons for stability
The researchers attribute the high thermostability to the physical stability of the NLC, and the protection it confers against RNase activity on the RNA because of electrostatic forces between the negative RNA backbone and the cationic lipid of the NLC core.
Secondly, the capability to undergo lyophilization is a key factor. The use of sucrose and similar lyoprotectants substitute the water in hydrogen bonds with the system’s own components, or provide a protective rigid sugar matrix, restricting enzymatic activity. Lyophilization is difficult with LNPs, since freeze-drying necessarily disrupts their aqueous core-lipid bilayer structure.
What are the implications?
We demonstrate that a safe and effective NLC-based RNA vaccine delivery system has greatly increased thermostability over current LNP formulations. This NLC-based delivery technology represents a significant advance for RNA vaccines with potentially paradigm-shifting implications on vaccine manufacture, storage, distribution, and overall cost due to its thermostable properties.”
Further optimization would allow the formulation to resist still more extreme temperatures or abrupt shifts in temperatures, as may be expected when vaccines are shipped globally.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Gerhardt, A. et al. (2021). A Thermostable, Flexible RNA Vaccine Delivery Platform for Pandemic Response. bioRxiv preprint. doi: https://doi.org/10.1101/2021.02.01.429283, https://www.biorxiv.org/content/10.1101/2021.02.01.429283v1
- Peer reviewed and published scientific report.
Gerhardt, Alana, Emily Voigt, Michelle Archer, Sierra Reed, Elise Larson, Neal Van Hoeven, Ryan Kramer, Christopher Fox, and Corey Casper. 2022. “A Flexible, Thermostable Nanostructured Lipid Carrier Platform for RNA Vaccine Delivery.” Molecular Therapy - Methods & Clinical Development 25 (June): 205–14. https://doi.org/10.1016/j.omtm.2022.03.009. https://www.cell.com/molecular-therapy-family/methods/fulltext/S2329-0501(22)00039-0?.