A team of scientists from India has recently tested the efficacy and immunogenicity of two different concentrations of a plasmid DNA-based vaccine candidate ZyCoV-D. The findings reveal that the vaccine candidate administered via Needle Free Injection System (NFIS) can significantly induce the production of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein-specific IgG and neutralizing antibodies for a prolonged period. The study is currently available on the bioRxiv* preprint server.
The recent outbreak of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has placed a heavy burden on many countries' socioeconomic and healthcare structures.
Several efforts have been made to control the pandemic situation, including the repurposing of antiviral medicines and the development of potential vaccines and therapeutic monoclonal antibodies.
Currently, two mRNA-based vaccines have received approval for emergency use in the USA, UK, Canada, and European Union, and two adenovirus-based replication incompetent viral vaccines are rolling out in the UK, Russia, and India.
However, because of the limited availability, COVID-19 vaccination programs are primarily focusing on immunizing healthcare and other front-line workers and at-risk individuals. Thus, to increase the vaccine coverage to the entire world population, more vaccines with good efficacy and safety are urgently needed.
In the current study, the scientists have assessed the SARS-CoV-2 infection protecting efficacy of a DNA-based vaccine candidate ZyCoV-D in rhesus macaques. Cadila Healthcare Limited, India have developed the vaccine candidate. They tested two different doses of the vaccine candidate (1 mg and 2 mg) and two different administration routes (NFIS and intradermal syringe needle injection).
Important observations
The scientists tested the efficacy of the vaccine candidate in inducing the production of viral spike S1 domain-specific IgG and neutralizing antibodies during the immunization and viral challenge phases.
Specifically, they observed that 1 mg and 2 mg doses of the vaccine candidate administered via NFIS and syringe needle, respectively, could induce immune responses in only a limited number of monkeys, whereas 2 mg dose administered via NFIS could significantly induce the production of spike S1-specific IgGs and neutralizing antibodies during the immunization and viral challenge phases. This indicates the importance of vaccine dose and delivery system in inducing desired immune responses.
According to the scientists, the vaccine candidate's increased immunogenicity under the 2 mg via NFIS regimen could be due to the known fact that the efficacy of DNA vaccines increases dose-dependently and that the needle-free delivery systems can further improve the efficacy by increasing either vaccine dispersion or local inflammation.
A robust antibody response induced by the 2 mg via NFIS regimen of the vaccine candidate was observed up until 14 weeks, further increasing in response to viral challenge and continued to 15 days post-infection. With the same regimen of the vaccine candidate, robust neutralizing antibody response was observed from 6 weeks, which continued to increase throughout the immunization phase.
A reduction in viral load was observed by day 7 post-infection in the throat and nasal samples collected from immunized monkeys. Complete viral clearance was observed by day 14 post-infection.
In addition, the 2 mg via NFIS regimen of the vaccine candidate was found to significantly increase the levels of lymphocytes and cytokines (IL-6 and IL-5), indicating a robust induction of host immune responses by the vaccine candidate.
A transient reduction in CD8+ T cell population was observed at days 1 and 3 post-infection. As suggested by the scientists, such a reduction in T cell level could be due to a reduction in blood levels of lymphocytes caused by SARS-CoV-2 infection.
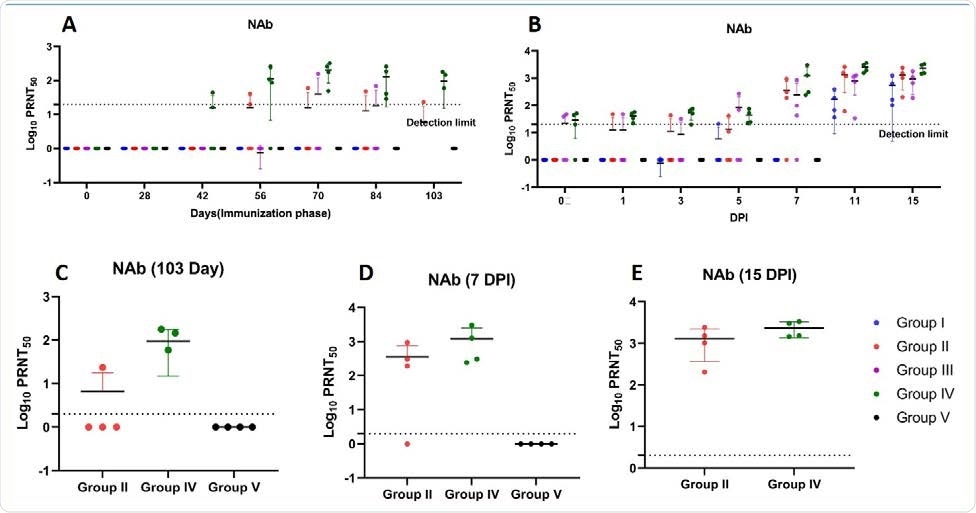
Neutralizing antibody response in rhesus macaques (A) NAb titers in animals of group I, II, III, IV and V from 0 to 103 days of immunization period (B) NAb titers in animals of group I, II, III, IV and V at 0, 1, 3, 5 7, 11 and 15 DPI (C) Peak NAb titers at day 103 of immunization phase of group II, IV and V (D) Peak NAb titers at 7 DPI of groups II, IV and V (E) Peak NAb titers at 15 DPI of group II and IV. The statistical significance was assessed using the Kruskal-wallis test followed by the two tailed Mann-Whitney test between two groups; p-values of less than 0.05 were considered to be statistically significant. The dotted line on the figures indicates the limit of detection the assay. Data are presented as mean values +/- standard deviation (SD). Statistical comparison was done by comparing the vaccinated group with the placebo group as control. Group I = blue, group II = orange, group III = purple, group IV = green and group V = black, number of animals = 4 animals in each group.
Study significance
The study findings reveal that 2 mg dose of the DNA-based vaccine candidate ZyCoV-D administered via NFIS is highly efficient in inducing both binding and neutralizing antibody titers in monkeys, indicating the immunogenic efficacy of the vaccine candidate.
The vaccine candidate is also effective in reducing the viral load in monkeys infected with SARS-CoV-2, indicating the vaccine candidate's protective efficacy.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources