RNAssist (Cambridge UK) and Rapid Labs Ltd (Colchester, UK) have launched the virusPHIX™ range of virus inactivating and RNA stabilising transport media, following successful SARS-CoV-2 inactivation testing carried out by Public Health England (PHE).
virusPHIX™ is a novel RNA stabiliser and virus inactivation medium based on technology developed by RNAssist Limited (Cambridge, UK). It has been shown to stabilise viral RNA for up to 1 year at 20°C, and has successfully inactivated all tested viruses and bacteria to date including SARS-CoV-2, Hepatitis B, Vaccinia, FIV, BVDV, Dengue, Zika, Influenza A and M. tuberculosis.
Unlike other virus transport mediums, virusPHIX™ does not contain guanidine, a highly toxic substance than can produce hydrogen cyanide when mixed with household cleaning products such as bleach. virusPHIX™ is a completely non-toxic, non-volatile reagent that can be used safely in a home testing environment and shipped for PCR analysis. The virusPHIX™ range of products are manufactured and sold worldwide by Rapid Labs Limited (Colchester, UK). With over 200 evaluators around the world, virusPHIX™ is a popular choice for RNA stabilisation thanks to its compatibility with most front-end RNA purification kits.
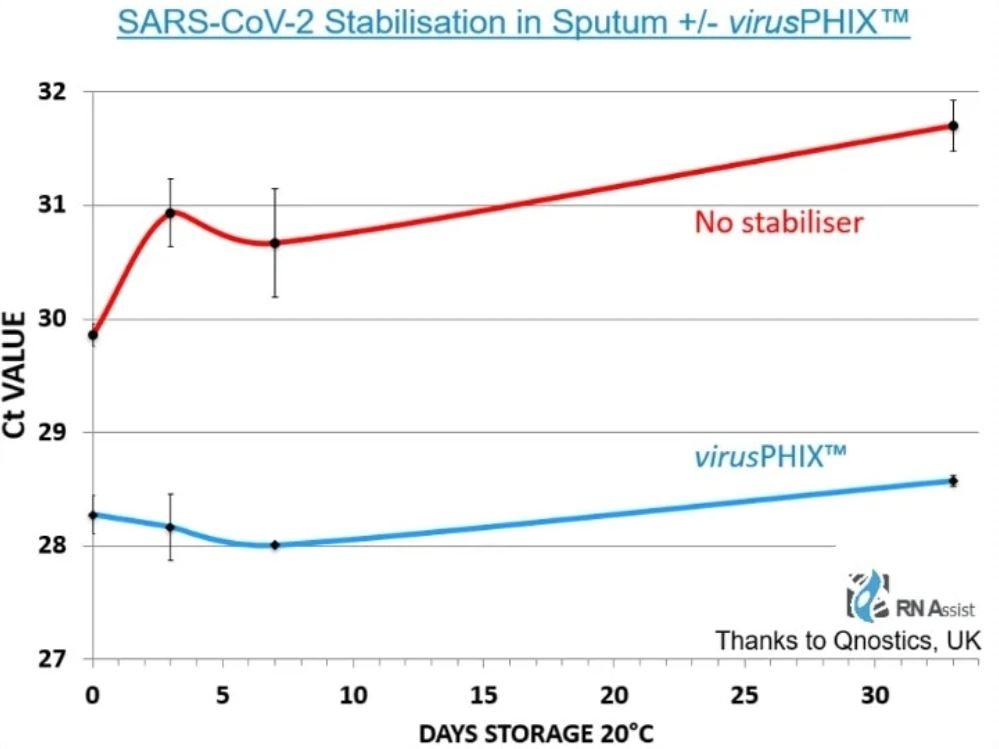
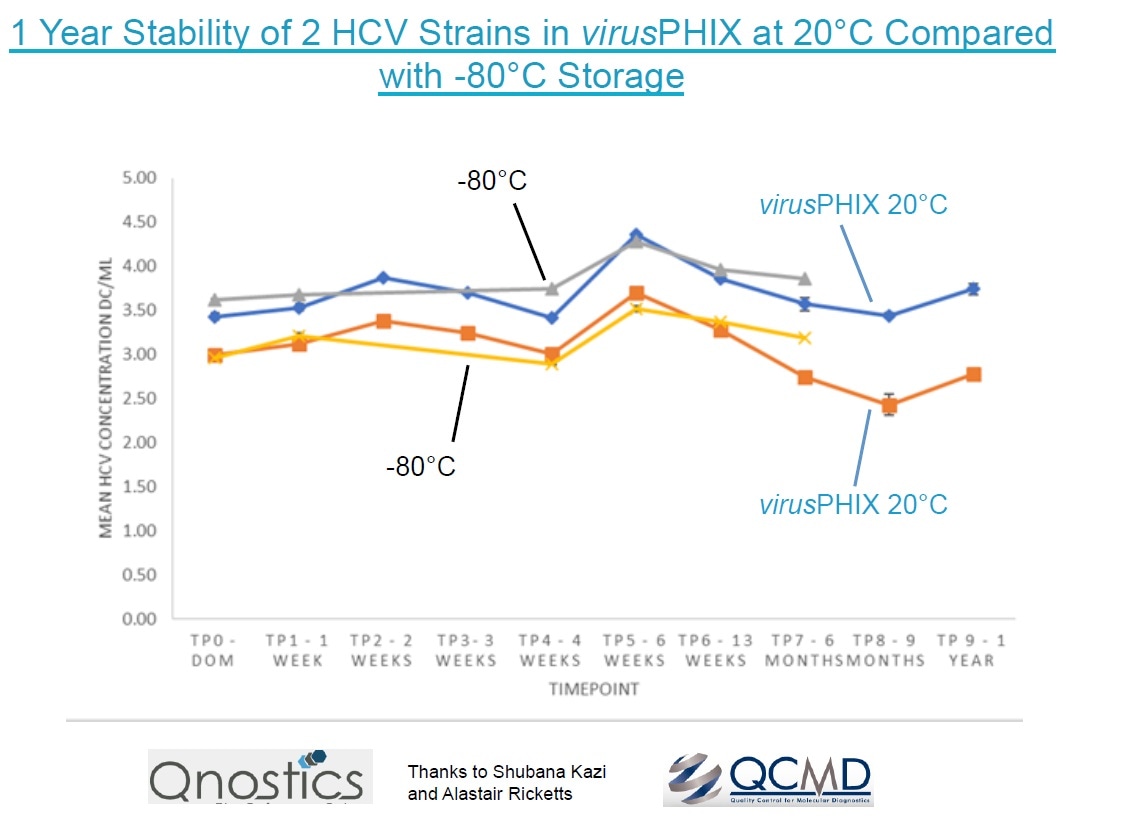
Public Health England and The Roslin Institute have demonstrated inactivation of SARS-CoV-2 within 10 minutes at 20°C. Qnostics UK have also demonstrated SARS-CoV-2 RNA stabilisation for 33 days at room temperature, and 12-month stability of 2 HCV strains at 20°C.
The virusPHIX™ range features 3 reagents suited to different applications:
- virusPHIX+™ for swab and saliva testing
- virusPHIX-LV™ (lower viscosity) for swab testing on automated liquid handling platforms (Ortho, Hamilton and Tecan)
- virusPHIX-P9™ for swab and saliva testing, especially when it is necessary to add sample directly into an RT-LAMP, LamPORE or RT-PCR assay without RNA purification.*
*virusPHIX-P9™ has been demonstrated to be directly compatible with LAMP assays. Please contact [email protected] to find out more.
Public Health England SARS-CoV-2 Inactivation Studies
PHE evaluated the reduction in virus titre SARS-CoV-2 spiked samples (tissue culture fluid) and presence of SARS-CoV-2 over a series of cell passages:
|
Treatment
|
Test 1: Reduction in SARS-CoV-2 Virus Titre
|
Test 2: Cell Passage
|
|
virusPHIX+™, 10 minutes
|
≥6.4 log10
|
Virus not detected
|
|
virusPHIX+™, 30 minutes
|
≥7.1 log10
|
Virus not detected
|
|
PBS Control, 10 minutes
|
None
|
Virus detected
|
|
PBS Control, 30 minutes
|
None
|
Virus detected
|
|
|
|
|
|
virusPHIX-LV™, 10 minutes
|
≥5.1 log10
|
Not tested
|
|
virusPHIX-LV™, 30 minutes
|
≥6.0 log10
|
Not tested
|
|
PBS Control
|
None
|
Not tested
|
|
|
|
|
|
|
|
|
|
virusPHIX-P9™, 10 minutes
|
≥4.4 log10
|
Not tested
|
|
virusPHIX-P9™, 30 minutes
|
≥4.8 log10
|
Not tested
|
|
PBS Control
|
None
|
Not tested
|
The full PHE Reports can be found online at https://assets.publishing.service.gov.uk.
More information about the virusPHIX™ range can be found in their online brochure: https://www.rapidlabs.co.uk/wp-content/uploads/virusPHIX-range-brochure-V4.pdf.
For ordering enquiries please contact [email protected].