In December 2019, a ‘viral pneumonia of unknown cause’ was identified in Wuhan, China, which was later identified as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
SARS-CoV-2 infection causes COVID-19 (coronavirus disease 2019) and is characterized predominantly by cough, fever, fatigue, and acute respiratory distress syndrome (ARDS) associated with high mortality rates.
On March 11, 2020, the World Health Organization (WHO) officially declared the coronavirus outbreak to be a pandemic. To date, the SARS-CoV-2 infection has caused over 106 million confirmed cases. According to the WHO, it has caused over 2.3 million deaths.
Writing in the International Journal of Nanomedicine, Professor Dongki Yang, from the Department of Physiology, Gachon University, South Korea, has reviewed the available knowledge on the coronaviruses, current approaches to tackle the newly emerged SARS-CoV-2 infection, and the innovative application of nanotechnology in the COVID-19 pandemic.
Coronaviruses
Coronaviruses (CoVs) are RNA viruses, 27–32 kb (kilobases) in size, and belong to the Coronaviridae family. These viruses infect humans and other animals. These fast-evolving human coronaviruses (because of their high genomic nucleotide recombination rates) give rise to bronchiolitis, pneumonia, and also the common cold.
There are currently seven human coronaviruses known: (HCoV-229E, HCoV-NL63, HCoV-HKU1, HCoV-OC43, Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV, and SARS-CoV-2).
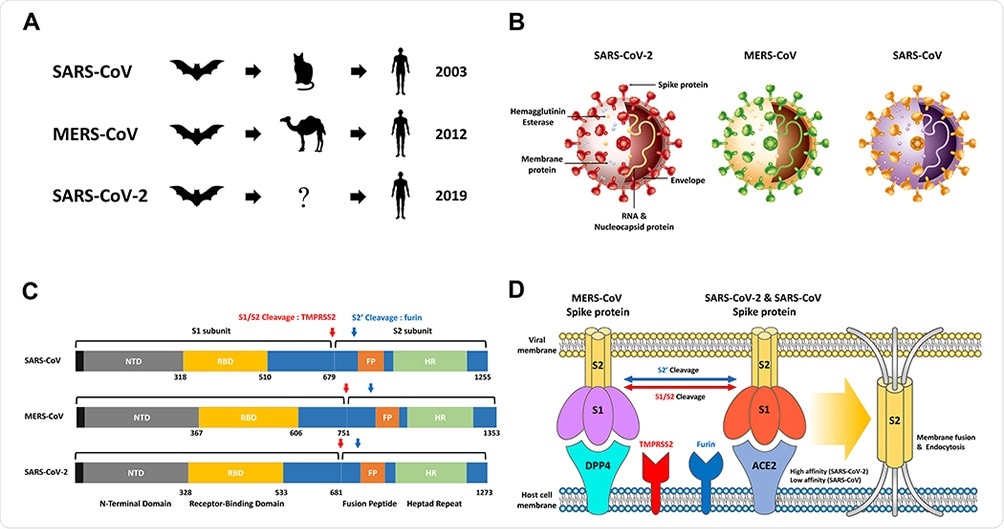
SARS-CoV, MERS-CoV, SARS-CoV-2 overview. (A) The origin of SARS-CoV, MERS-CoV and SARS-CoV-2 is widely known as bats as native hosts. While SARS-CoV and MERS-CoV have been shown to be intermediary hosts in civets and camels, SARS-CoV-2 can infect humans through an as-yet-unknown intermediate host. After animal infection, SARS‐CoV‐2 has spread rapidly worldwide to date, mainly through continuous human-to-human transmission. (B) Schematic structure of SARS-CoV-2, MERS-CoV and SARS-CoV including Hemagglutinin Esterase, Membrane protein, RNA & Nucleocapsid protein, Envelope, and Spike protein. (C) Comparison of the S proteins of SARS-CoV, MERS-CoV and SARS-CoV-2. NTD, RBD, FP, HR, and Cleavage site by TMPRSS2 and furin. (D) The MERS-CoV, SARS-CoV, and SARS-CoV-2 S proteins bind to ACE2 and DPP4, which act as receptors present in host cells. In order for the S2 domain in the virus to be fused to the host cell membrane to cause endocytosis, the process of cutting at two sites (S1/S2 and S2’) through the proteases Furin and TMPRSS2 are essential. SARS-CoV-2 has a much higher affinity than binding affinity to SARS-CoV S protein and ACE2, resulting in a high infection rate.
These human coronaviruses are one of the top 10 known viruses fatal to human beings. The mortality rate of SARS-CoV is up to 10%, and that of MERS-CoV is 36%.
The structural and functional physiology of the viruses SARS-CoV, MERS-CoV, and SARS-COV-2 are detailed in the review, followed by therapeutic strategies.
Because of the prevalent mild-to-no symptoms in the case of the SARS-CoV-2, the asymptomatic transmission of the virus has contributed to the fast and wide spread of the virus. Thus, vaccines are essential.
At an unprecedented pace, vaccines have been developed; likewise, scientists have also designed diverse therapeutics to fight this infection.
In this regard, nanotechnology presents many opportunities to fight the virus: nano-based formulations, nano-vaccine metastasis platforms, and nano-drugs.
Therapeutic Strategies
Several antiviral drugs approved for other applications have some effect on SARS-CoV-2, but the results were different in small-scale non-randomization trials. These include remdesivir, which was developed as an experimental drug against Ebola virus (EBOV) during the Ebola epidemic in West Africa, chloroquine (CQ) and hydroxychloroquine (HCQ) for malaria, and lopinavir/ritonavir (LPV/r), which is used as an acquired immunodeficiency syndrome (AIDS) treatment.
The spike (S) protein and ACE2 (Angiotensin-Converting Enzyme 2) interaction inhibitors and the neutralizing antibodies prevent virus transmission and immediate protection.
In immunotherapy, Tocilizumab (a monoclonal antibody against IL-6) blocks the signal transduction that triggers the inflammatory responses; IL-6 is one of the leading causes of inflammation, leading to severe outcomes in COVID-19 disease. However, the cost and safety aspects can hinder the use of Tocilizumab in COVID-19 treatment, writes the author.
Many clinical trials are testing the convalescent plasma (CP) therapy, another effective method for COVID-19 treatment globally.
RNA interference (RNAi) is also a new therapeutic strategy for SARS-CoV because it can degrade specific mRNAs.
Preventive Vaccination Strategies
Data from the vaccine development for SARS-CoV and MERS-CoV helped develop vaccine candidates for SARS-CoV-2. In an unparalleled achievement in the history of vaccine development, inactivated or live-attenuated vaccines and recombinant vaccines have been developed with a high neutralization ability. Effective vaccines against the SARS-CoV-2 variants are now being administered the world over - beginning the fight to eradicate the pandemic virus.
Application of Nanotechnology in COVID-19 Therapeutics
Nanoparticles and viruses are comparable in size and function. Various treatments using nanotechnology have been developed and commercialized for common viral infections, such as IAV and IBV (influenza virus), EBOV (Ebola), HIV1 and 2 (human immunodeficiency viruses), HSV1 and 2 (herpes simplex virus), HBV and HCV (hepatitis B and C), and HuNoV.1 (human noroviruses).
“The accumulated advancements in these virus-fighting nanotechnologies can play an important role in taking SARS-CoV-2 treatment and vaccine development to the next level.”
Nanomedicines evolve as effective alternatives to vaccine technologies. While presently vaccines are being delivered to people, nanotechnology and nanomedicine are presented in the review as new therapeutic technologies and approaches that can soon have a clinical impact.
Professor Dongki Yang discusses theranostic nanoparticles, nanoparticles delivery therapy, nanotechnology-based diagnosis, nanotechnology-based vaccine development subunit vaccines, other vaccines (nucleic acid vaccines and NP-based vaccines) and inactivation of SARS-CoV-2 in the external environment using nanotechnology.
Dexamethasones, a COVID-19 therapeutic agent introduced via various nano-formulations, has led to a significant turn in the treatment of COVID-19.
Also, Pfizer’s liposomal mRNA vaccine (BNT162b) is a considerable achievement of nanomedicine, containing lipid-nanoparticle (LNP) formulated nucleoside-modified mRNA encoding SARS-CoV-2 spike glycoprotein-derived immunogens.
Also, technologies that employ nanomaterials, such as silver nanoparticles, copper or copper oxide nanoparticles, and gold nanoparticles, prevent and control COVID-19 by deactivating the SARS-CoV-2 in the environment. In short, nanotechnology and nanomedicine can be suitable alternatives to this change in the research and development paradigm, Professor Yang recommends.