Researchers in the United States have conducted a study showing that the Moderna mRNA-1273 vaccine effectively protects against recently emerged variants of severe acute respiratory syndrome coronavirus 2 – the agent that causes coronavirus disease 2019 (COVID-19).
The team compared the neutralization activity of blood taken from infected and vaccinated individuals against a panel of SARS-CoV-2 variants, including the B.1.1.7 that emerged in the UK.
The team – from Emory University School of Medicine in Atlanta, the University of Texas Medical Branch, and the COVID-19 Neutralization Study Group – found that antibodies generated by both infected and vaccinated individuals effectively neutralized all of the variants.
These findings support the notion that in the context of the UK variant, vaccine-induced immunity can provide protection against COVID-19,” says Mehul Suthar and colleagues.
However, as further variants continue to emerge, it will be essential to assess their impact on the potency of neutralizing responses following both infection and vaccination, warns the team.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The SARS-CoV-2 infection process
In order to infect cells, SARS-CoV-2 uses a surface viral structure called the spike protein to bind to the host cell receptor angiotensin-converting enzyme 2 (ACE2).
The spike protein is the primary target of neutralizing antibodies and levels of these antibodies correlate with protection against COVID-19.
Neutralizing antibodies are generated within 10 days of symptom onset and studies have shown that they are maintained for at least 8 months.
Similarly, Moderna’s mRNA-1273 vaccine generates neutralizing antibodies that are detectable for at least 119 days.
However, the recent emergence of SARS-CoV-2 variants, including the B.1.1.7 strain that has arisen in the UK, has sparked concerns regarding the breadth of these neutralizing antibody responses.
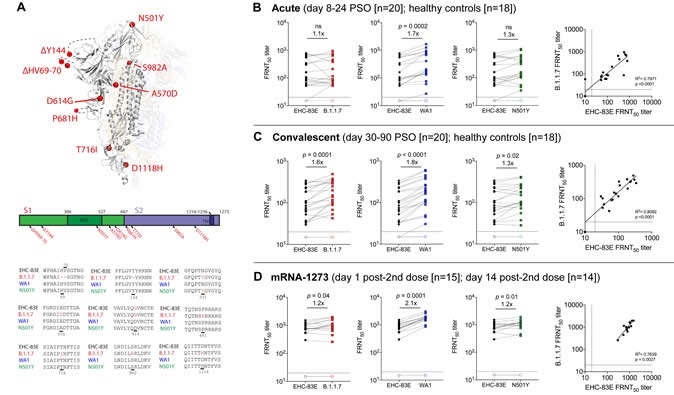
Neutralizing antibody responses against the SARS-CoV-2 B.1.1.7 viral variant. Shown are data from the following cohorts based on natural infection: 20 acutely infected COVID-19 patients (8-24 days PSO; closed symbols), 20 convalescent COVID-19 individuals (30-90 days PSO, closed symbols) 18 healthy controls (open symbols); and individuals that received 100 ug of mRNA-1273 (18 to 55 years old) on day 1 post-2nd dose (15 participants; open symbols) and day 15 post-2nd dose (14 participants; closed symbols). A schematic of the amino acid changes within the spike protein are shown between the SARS-CoV-2 variants (Panel A); The 50% inhibitory titer (FRNT50) on the focus reduction neutralization (FRNT) assay for the EHC-83E (black), B.1.1.7 (red), WA1 (blue) and N501Y (green) SARS-CoV-2 variants and correlation plots between EHC-83E and B.1.1.7 viruses are shown for the acutely infected COVID-19 patients (Panel B), convalescent COVID-19 individuals (Panel C), and mRNA-1273 vaccinated individuals (Panel D). Statistical significance was determined using a Wilcoxon paired t-test. The GMT fold change for the respective isolates relative to EHC-83E is shown in each of the plots.
What did the researchers do?
Suthar and colleagues compared the neutralization potency of sera from 20 patients with acute infection, 20 recovered individuals, and 14 healthy vaccinees, against a panel of SARS-CoV-2 variants.
Symptom onset had occurred between 8 and 24 days previously among the 20 acutely infected patients and between 30 and 90 days previously among the convalescent patients.
The immunized participants (aged between 18 and 55 years) had received two injections of Moderna’s mRNA-1273 vaccine at a dose of 100µg, with the second dose administered 14 days previously.
The panel of SARS-CoV-2 variants included an early variant isolated from an individual in Washington (WA1); a later D614G variant isolated from a patient in Georgia in March 2020 (EHC-083E), and a B.1.1.7 variant isolated from a patient in California.
The team also included a recombinant SARS-CoV-2 virus containing a single point mutation within the spike protein at position 501 (N501Y).
Levels of neutralizing antibodies were measured using a live-virus Focus Reduction Neutralization Test.
What did they find?
The findings showed that antibodies were effective at neutralizing all variants, whether they were generated following natural infection or vaccination.
The team observed no significant reduction in levels of neutralizing antibodies against any of the variants.
“These results show that neutralizing antibody titers following natural infection or vaccination are effective against the UK variant (B.1.1.7) and viral strains containing single point mutations at positions 501 and 614 within the spike protein,” writes the team.
The researchers say the findings suggest that in the context of the UK variant, vaccine-induced immunity can provide protection against COVID-19.
“As additional SARS-CoV-2 viral variants continue to emerge, it is crucial to monitor their impact on neutralizing antibody responses following infection and vaccination,” they conclude.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.