The rapid spread of newly emerging variants seems to put the end of the coronavirus disease 2019 (COVID-19) pandemic still farther away, with more infective and virulent strains now observed. The rollout of the earliest vaccines to be approved has, however, raised hopes that population immunity can be achieved considerably earlier than otherwise thought.
The role of a vaccine against infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the virus that is responsible for COVID-19 – is expected, or rather hoped, to be two-fold. First, it should elicit protective neutralizing antibodies against the virus, typically inhibiting viral spike-host receptor entry. Secondly, it should reduce the viral load in case breakthrough infection does occur following vaccination. This would be of immense help in breaking the chain of transmission.
A new study by researchers in Israel, published on the medRxiv* preprint server, reports that this could indeed be the case with a newly approved mRNA vaccine, BNT162b2. Not only does the vaccine reduce the incidence of disease by 95% in vaccinated individuals, at seven days from the completion of the vaccination, but it reduces the viral load fourfold in breakthrough infections.
Several countries have begun to vaccinate their people on a phased priority basis, hoping to complete the vaccination of a significant share of the population within a short period. This is expected to reduce the basic reproduction number of the virus, the number of secondary infections arising from a single case.
Mechanisms of vaccine protection
The mechanisms by which such an outcome can be achieved include a reduction in the number of susceptible individuals, a reduction in the viral load, and thus, reduced viral shedding from people who develop the infection after vaccination. The result is thus, in all scenarios, a reduction in the number of new infections.
Vaccination and viral loads
There is little data on the effect of vaccination on viral loads. The current study sheds light on this outcome of vaccine administration.
The researchers examined the positive results of reverse transcriptase-polymerase chain reaction (RT-PCR) testing that assessed the presence of viral RNA encoding three important SARS-CoV-2 viral genes – the envelope and nucleocapsid structural proteins, and the RNA-dependent RNA polymerase (RdRp) enzyme.
Among the positive PCR tests, the cycle threshold (Ct) values over time reflected a substantial reduction in average viral load at 12 days post-vaccination. This is the time point associated with the beginning of vaccine-related protection, following the first dose.
Ct values vary with period since vaccination
When daily Ct values were averaged for each day from vaccine administration, they observed that the values were higher over post-vaccination days 12-28 than days 1-11. The differences for the RdRp, E and N gene were 2.1, 1.9 and 1.6, respectively.
Compared to Ct values from the positive tests of unvaccinated patients, those from vaccinated patients were similar over post-vaccination days 1-11, among groups matched for age and sex, and the date of sampling.
After this period, that is, post-vaccination days 12-28, Ct values increased across all three target genes in the vaccinated group. Overall, considering all infections occurring in both vaccinated and non-vaccinated groups, the researchers found that Ct values increased by 1.6 for the N gene to 2.3 for the RdRp gene if the infected individual was vaccinated 12 days or more before the sample.
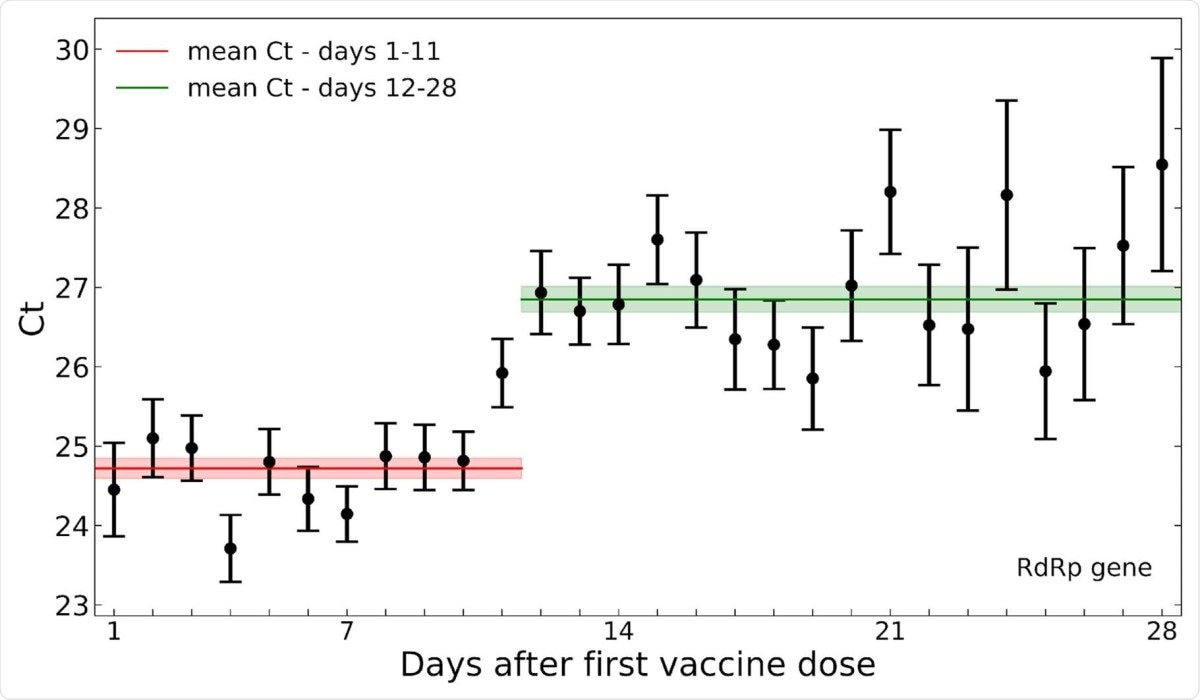
Decreased SARS-CoV-2 viral load after 12 days post-vaccination. Mean Ct values of the RdRp gene for positive tests following vaccination are plotted by the post-vaccination day in which the sample was taken. Error bars indicate the standard error of the mean.
What are the implications?
Given that a Ct value difference of 1 corresponds to almost twofold higher viral particles per sample, the differences found in this study indicate that the viral load in infected vaccinated individuals is almost 3 to 4.7 times higher compared to that in vaccinated individuals who have a breakthrough infection within 12-28 days after vaccination.
This means such patients would shed much lower levels of virus, be much less contagious, and would develop much milder disease. This is an important observation on vaccine impact, though the study design does not allow causality to be inferred.
Some caveats are expressed, namely, the possibility that there are significant behavioral differences between the vaccinated and non-vaccinated cohorts, which could affect their overall health or chances of being tested. Secondly, the viral variants in different population segments may be different, associated with variations in viral loads.
Thirdly, positive tests following vaccination may have a higher proportion of long-term infections with a low viral load, resulting from an infection that occurred before vaccination. If so, longitudinal studies will capture further changes in the mean viral load in post-immunization infections.
Finally, the use of oronasopharyngeal specimens fails to distinguish viral loads in the nose from that in the mouth, though the latter represents viral shedding and infectiousness more accurately.
The researchers await more studies with longer periods of follow up, to report variations in post-vaccination viral loads and viral shedding, with different variants and vaccines. Nonetheless, these findings can help fine-tune the epidemiological studies dealing with the early impact of the vaccines on viral spread.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Levine-Tiefenbrun, M. et al. (2021). Decreased SARS-CoV-2 viral load following vaccination. medRxiv preprint. doi: https://doi.org/10.1101/2021.02.06.21251283, https://www.medrxiv.org/content/10.1101/2021.02.06.21251283v1
- Peer reviewed and published scientific report.
Levine-Tiefenbrun, Matan, Idan Yelin, Rachel Katz, Esma Herzel, Ziv Golan, Licita Schreiber, Tamar Wolf, et al. 2021. “Initial Report of Decreased SARS-CoV-2 Viral Load after Inoculation with the BNT162b2 Vaccine.” Nature Medicine 27 (5): 790–92. https://doi.org/10.1038/s41591-021-01316-7. https://www.nature.com/articles/s41591-021-01316-7.