The ongoing coronavirus disease 2019 (COVID-19) pandemic continues to ravage the globe. To date, over 106.56 million people have been infected with the disease's causative agent: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Developing effective and safe vaccines and therapeutics will be crucial in turning the tide of this global health crisis. Currently, the United States Food and Drug Administration (FDA) has granted an emergency use authorization (EUA) for some vaccine candidates as well as monoclonal antibodies (mAbs) and convalescent plasma therapies.
These countermeasures to combat the pandemic have a shared feature: they all involve neutralizing antibodies that target the spike protein (S) of SARS-CoV-2. The spike protein acts as a key that binds with the human cell's angiotensin-converting enzyme 2 (ACE2) receptor, where the virus enters to infect the cell.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Now, a new study, published on the medRxiv* preprint server, shows a fully human immunoglobulin (IgG) that potently inhibits SARS-CoV-2 infection. The researchers at Washington University in Saint Louis, USA, believe that the discovery could become a potential treatment for COVID-19, which has now claimed over 2.32 million people.
The threat of new variants
Concern has been mounting that the emergence of highly infectious new variants, like those reported in the UK and South Africa, may threaten the efficacy of vaccines and mAbs. Genomic analysis of circulating SARS-CoV-2 variants has 51 identified mutations in the S protein, including the E484L, that evade convalescence plasma and mAb neutralization in cell culture-based assays.
The new study aims to determine an alternative way or treatment that cannot be affected by amino-acid substitutions and deletions in spike protein (S).
To arrive at the study findings, the team used a fully-human, polyclonal anti-SARS-CoV-2 immunoglobulin Tc-hIgG74 SARS-CoV-2, called SAB-185. This is produced from Tc bovines hyperimmunized with two doses of plasmid deoxyribonucleic acid (DNA) encoding the SARS-CoV-2 Wuhan-Hu-1 strain Spike (S) gene. The team then followed these with repeated doses of recombinant S protein.
The study findings showed that the neutralizing polyclonal human IgG produced in transchromosomic bovines effectively neutralizes SARS-CoV-2 and VSV-SARS-CoV-2 chimeras containing one or two S mutations present in current circulating variants.
The potency of the SAB-185 with the combination of the mutations E484L and N501Y shows that the hyperimmune IgG is unlikely to escape neutralization.
In phase 1 clinical trials of SAB-185, the team found that it is safe and well-tolerated with a potent neutralization efficacy in vitro. The use of these novel therapeutics can pave the way to fight the pandemic.
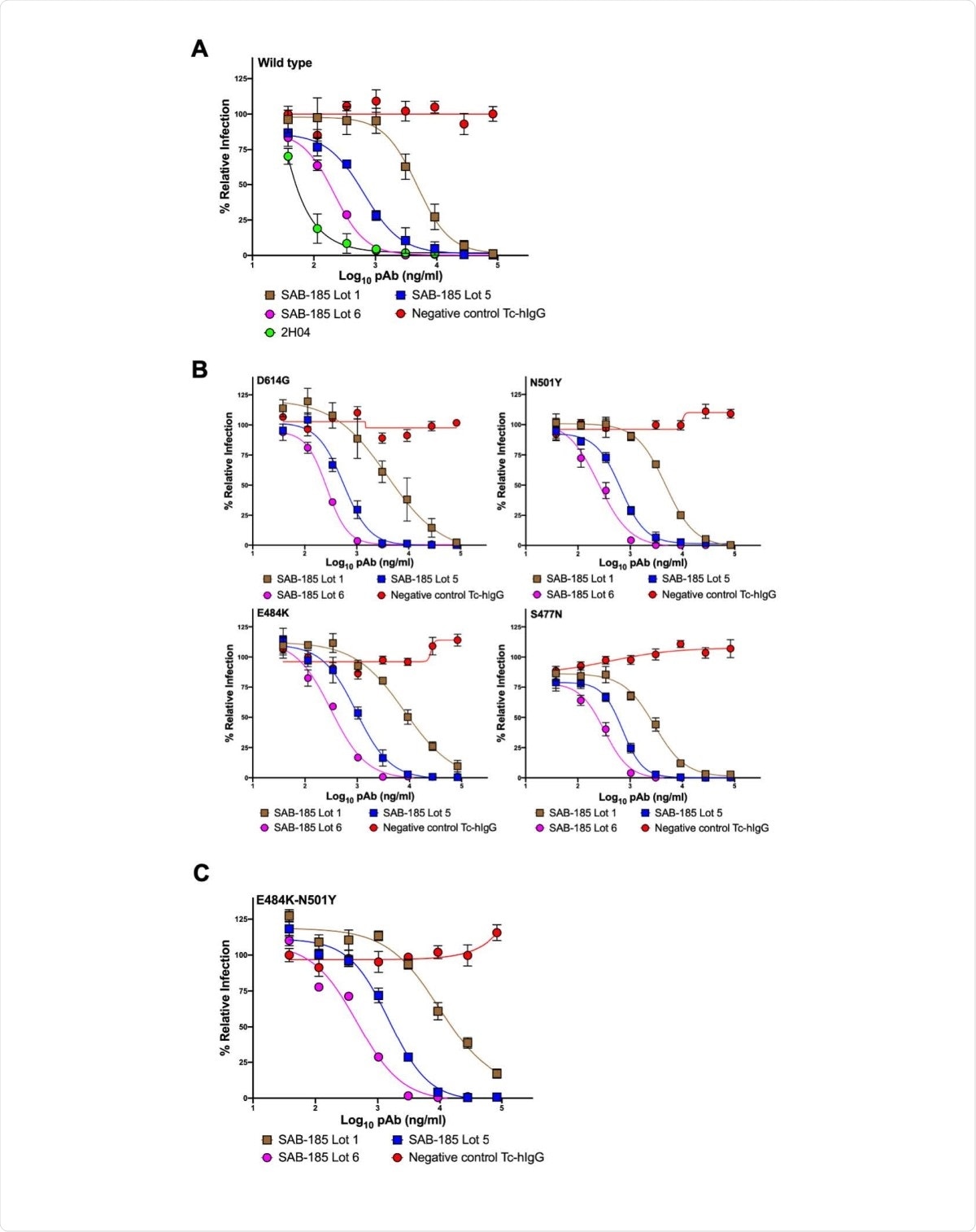
Neutralization of VSV-SARS-CoV-2 mutants by polyclonal antibody. (A) three SAB-185 pAbs were tested for neutralization of wild-type and (B) single amino acid substitution and (C) two amino acid substitution mutant VSV-SARS-CoV-2 (n = 4). Error bars represent the SEM. Data are representative of four independent experiments.
This new method shows potential in developing new immunotherapeutics, which can help combat the ongoing pandemic and potentially bring the lives of people worldwide back to normal.
This fully human immunoglobulin that potently inhibits SARS-CoV-2 infection may provide an effective therapeutic to combat COVID-19," the team concluded in the study.
The method, which produces human IgG transchromosomic cattle has been described in many emerging viruses worldwide, including the Ebola virus, Hantaan, and the Middle East coronavirus (MERS-CoV), a virus akin to SARS-CoV-2.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references:
- Preliminary scientific report.
Liu, Z., Wu, H., Egland, K., et al. (2021). Human immunoglobulin from transchromosomic bovines hyperimmunized with SARS-CoV-2 spike antigen efficiently neutralizes viral variants. medRxiv. doi: https://doi.org/10.1101/2021.02.06.430072, https://www.biorxiv.org/content/10.1101/2021.02.06.430072v1
- Peer reviewed and published scientific report.
Liu, Zhuoming, Hua Wu, Kristi A. Egland, Theron C. Gilliland, Matthew D. Dunn, Thomas C. Luke, Eddie J. Sullivan, William B. Klimstra, Christoph L. Bausch, and Sean P. J. Whelan. 2021. “Human Immunoglobulin from Transchromosomic Bovines Hyperimmunized with SARS-CoV-2 Spike Antigen Efficiently Neutralizes Viral Variants.” Human Vaccines & Immunotherapeutics, July, 1–10. https://doi.org/10.1080/21645515.2021.1940652. https://www.tandfonline.com/doi/full/10.1080/21645515.2021.1940652.