Vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are being rolled out worldwide to control the COVID-19 (coronavirus disease 2019) pandemic. Multiple vaccines are necessitated to address the variant epidemics and also overcome the challenges that may be posed by the current vaccines (such as high cost, or low immunogenicity).
In this context, a team of experts has tested the immune responses elicited by mucosal homologous plasmid and a heterologous immunization strategy using a plasmid vaccine and a Modified Vaccinia Ankara (MVA) expressing SARS-CoV-2 spike (S) and nucleocapsid (N) antigens. The research is published in the journal Vaccines.
The scientists describe the pre-clinical development of a mucosal, 2-dose heterologous vaccine candidate against the COVID-19 using the QAC (quil-A chitosan) adjuvant system. They showed that the pQAC/MVA-CoV-immunized mice efficiently neutralized the wild-type SARS-CoV-2.
They found that only the heterologous intranasal immunization strategy elicited neutralizing antibodies against SARS-CoV-2 in serum and bronchoalveolar lavage of mice. This finding suggests that this is a protective vaccine. They reported that this strategy led to the induction of type 1 and type 17 T-cell responses and polyfunctional T-cells expressing multiple type 1 cytokines (e.g., IFN-γ, TNFα, IL-2) in the lungs and spleens of the vaccinated mice.
The researchers found that the plasmid homologous vaccine strategy led to the induction of local mono and polyfunctional T-cells secreting IFN-γ.
The researchers noted that multiple vaccine constructs could be used together to immunize patients effectively.
In the study, the researchers have used 1) vectored (DNA and viral) vaccines - because plasmids encoding antigens can be developed within a few days with current rapid and inexpensive gene synthesis technologies, and 2) viral vectored vaccines like the Modified Vaccinia Ankara (MVA) strain - highly efficient scale-up production processes have been set-up already.
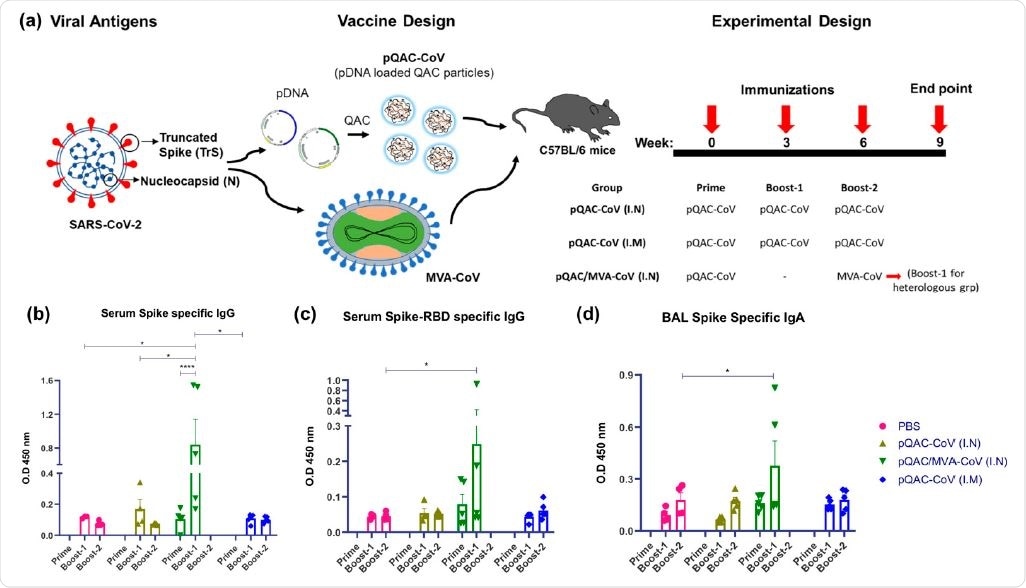
Generation of humoral immune responses in C57BL/6 mice following immunization with different vaccine constructs. (a) Outline for vaccine construct and immunization protocol using groups of C57BL/6 mice vaccinated with 3 doses of pQAC-CoV (I.N) or pQAC-CoV (I.M) with 3-week interval. Another group of C57BL/5 mice were vaccinated with pQAC-CoV (I.N) at week-0 followed by boost with MVA-CoV (I.N) at week-6. (b) ELISA titers of SARS-CoV-2 S-specific IgG in mice sera, (c) ELISA titers of SARS-CoV-2 spike receptor-binding domain (RBD)-specific IgG in mice serum and (d) ELISA titers of SARS-CoV-2 S-specific IgA in bronchoalveolar lavage (BAL), significance (*, p < 0.05, ****, p < 0.0001) was determined by two-way ANOVA. Data show mean � SEM.
While the DNA vaccines are temperature-stable (without needing cold-chain logistics), these DNA constructs are degraded in vivo by DNases. This leads to the inefficient uptake by antigen-presenting cells (APC) and thus causes low immunogenicity.
To overcome this challenge in using a DNA vaccine, the researchers have used an articulate delivery system such as the quil-A-loaded chitosan (QAC). Chitosan is a biodegradable natural polysaccharide that complexes with the DNA owing to its positive charge. The quil-A is a potent adjuvant with mild surfactant properties. The QAC particulate adjuvant system prolongs the release of the active plasmid expressing antigens.
In a previous study, the researchers showed that a 2-dose QAC encapsulated plasmid DNA (pQAC) encoding the nucleocapsid (N) gene against avian coronavirus elicited robust T-cell responses without complementing humoral responses.
With this observation, the researchers went on to develop a heterologous strategy with QAC encapsulated plasmid DNA (pQAC) prime, followed by an MVA boost, expecting a broader immune response. Both the pQAC plasmids (pQAC-CoV) and MVA vector (MVA-CoV) were designed to express the S (spike) and the N (nucleocapsid) antigens encoded by the SARS-CoV-2 from the early phase of the COVID-19 pandemic.
The researchers observed that the pQAC/MVA-CoV induced both systemic and local neutralizing antibodies in mice (when immunized intranasally), complemented by localized Th17 cellular responses. Moreover, they reported that the mice vaccinated with only plasmid vectors (pQAC-CoV) generated significant type 1 and type 17 (Tc17 or Th17) cellular responses. They discussed the local and systemic immune response in detail in the paper.
Based on the reported studies on the levels of anti-SARS antibodies, memory B cells and the T-cells, the researchers have noted that the vaccines relying solely on a neutralizing antibody response (that declines within 3 months after recovery) may not confer long-term protection against SARS-CoV-2 and other coronaviruses.
Keeping this in mind, the researchers have studied vaccines against the SARS-CoV-2 - that can induce both humoral and cellular immune response. Clearly, this may provide a durable protective immune response than vaccines that just focused on neutralizing antibodies.
“Currently, most experimental DNA vaccines are only amenable for intramuscular administration limiting mucosal immunity critically needed to reduce viral infection.”
With new infectious diseases, there is always a need for new vaccines. Vaccines engineered with processes that are well standardized enable their utility during a pandemic such as COVID-19. Customizable vaccine constructs offer highly flexible vaccination programs. Because the heterologous vaccination elicited both local and systemic humoral and T-cell immune responses, the researchers have postulated that the heterologous vaccine strategy might be better at providing sterilizing immunity against SARS-CoV-2.
Journal reference:
- Chandrasekar, S.S.; Phanse, Y.; Hildebrand, R.E.; Hanafy, M.; Wu, C.-W.; Hansen, C.H.; Osorio, J.E.; Suresh, M.; Talaat, A.M., Localized and Systemic Immune Responses against SARS-CoV-2 Following Mucosal Immunization. Vaccines 2021, 9, 132. https://doi.org/10.3390/vaccines9020132, https://www.mdpi.com/2076-393X/9/2/132