Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the betacoronavirus responsible for the coronavirus disease-19 (COVID-19) pandemic, has caused over 111 million infections globally and approximately 2.46 million deaths to date. SARS-CoV-2 infects host cells using the entry receptor angiotensin-converting enzyme 2 (ACE2) resulting in clinical outcomes ranging from asymptomatic infection to severe symptoms and death. While multiple host factors such as age and sex contribute to severe disease and adverse clinical outcomes, the massive immune response to the virus plays a key role, as shown by the therapeutic benefit offered by immunomodulatory agents, including corticosteroids.
However, the immunopathogenesis of COVID-19 is not yet fully clear. Identifying the host cells that facilitate viral entry and characterizing their response to infection is critical to understanding COVID-19 pathogenesis and improving treatment methods. Studies have shown that the nasal epithelium is an important entry point for the SARS-CoV-2 virus. Viral shedding from the nasopharynx contributes to the higher viral transmissibility and pathological features, including anosmia. Nasal epithelial cells are early viral target cells and can induce the systemic immune response, potentially influencing clinical outcomes.
All these factors emphasize the need to analyze host-virus interactions in nasal cells further. The ACE2 receptor is regulated by interferons (IFNs), which implies a complex relationship between tropism and IFN signaling. Type I and type III IFNs play a crucial role in antiviral innate immunity in humans and are implicated in SARS-CoV-2 defense. Susceptibility to severe or fatal COVID-19 has been shown to be associated with harmful variants in IFNAR genes and IFN-I blocking autoantibodies.
Comprehensive characterization of the SARS-CoV-2 response of human nasal epithelial cells
Researchers from the UK and Germany recently reported a comprehensive characterization of the SARS-CoV-2 response of human nasal epithelial cells, revealing a response dominated by IFN-I/IIIs. Also, recombinant IFN-I/III treatment was found to efficiently block the replication of SARS-CoV-2, which suggests that mucosal delivery of IFNs may be a good strategy for prophylaxis post-exposure. The study is published on the preprint server bioRxiv*.
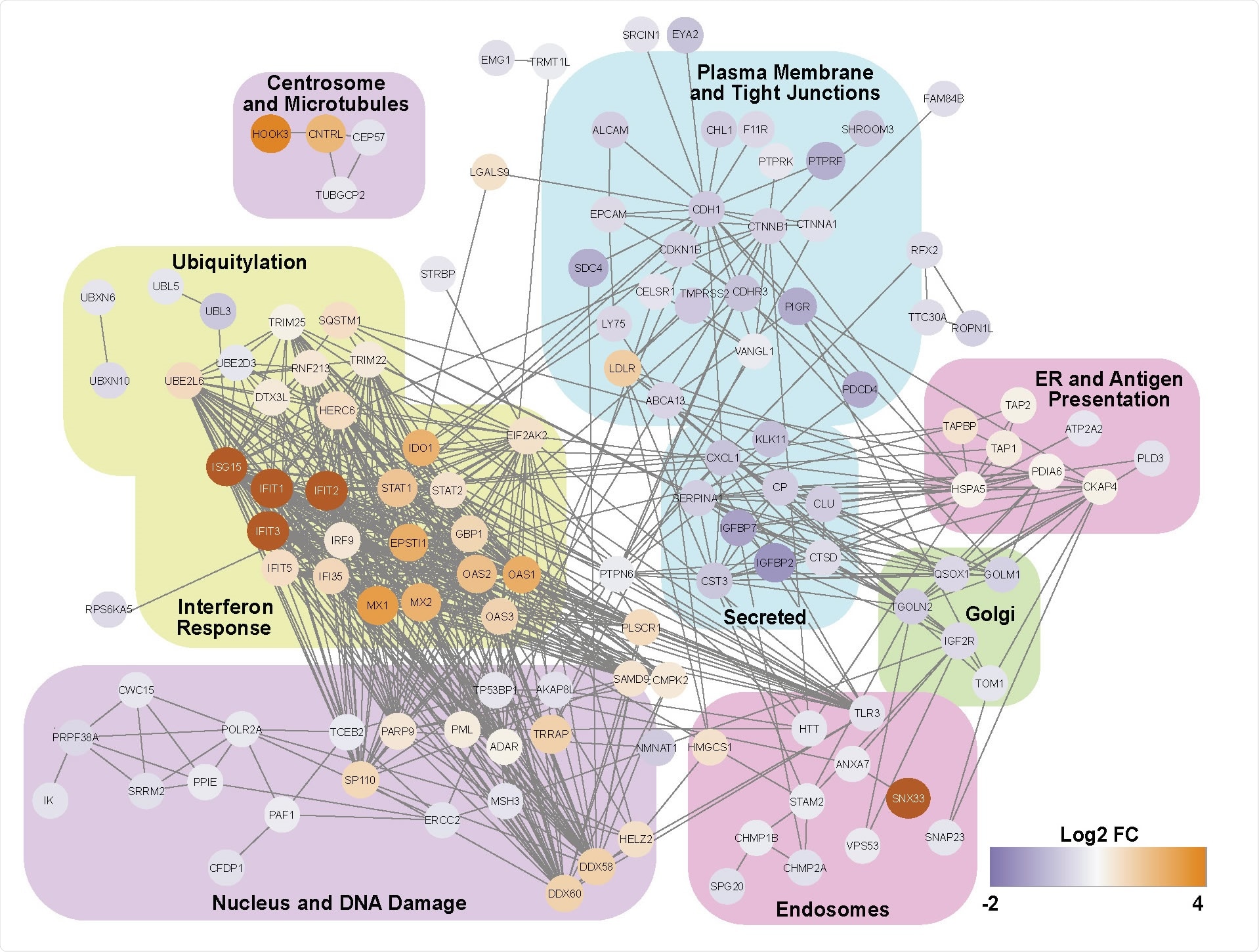
An ISG response dominates the proteome of SARS-CoV-2 infected nasal ALI cultures. Functional annotation network of differentially expressed proteins.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The researchers applied proteomics and single-cell RNA sequencing to a primary cell model of human nasal epithelium differentiated at an air-liquid interface. The results showed that the type I and III IFNs and interferon-stimulated gene products dominated the host response.
“Our finding that exogenous IFN-I or IFN-III can potently induce an antiviral state in nasal cells is consistent with its apparent protective effects in patients and in early phase clinical trials.”
This immune response was significantly delayed in onset compared to viral gene expression and hence failed to substantially impact SARS-CoV-2 replication. However, when IFNb or IFNl were provided pre-infection, they induced a good antiviral response that effectively restricted SARS-CoV-2 replication, thus preserving the integrity of the epithelial barrier. These findings suggest that nasal delivery of recombinant IFNs could be a potential chemoprophylactic strategy for SARS-CoV-2 infection.
“It is also worth noting our findings show that while all cell types contained SARS-CoV-2 protein, the intensity of immunodetection was greater in ciliated cells, which also contained more virion-like structures per cell.”
Challenges in vaccine coverage and effectiveness may necessitate the need for targeted chemoprophylactic strategies
This is a crucial observation that highlights the therapeutic value of chemoprophylaxis. It has already been tested in a small clinical trial in China but there was no control group in this trial, so it is difficult to assess the efficacy of this approach.
While immunization is the best approach for mass prevention of COVID-19, inadequate vaccine distribution, coverage, and effectiveness against mild or asymptomatic disease and the emergence of more infectious variants are serious challenges that will necessitate the targeted chemoprophylactic strategies to prevent viral transmission.
These strategies include post-exposure prophylaxis of contacts of COVID-19 patients that will help avoid the need for self-isolation and pre-exposure prophylaxis for high-risk encounters such as in healthcare facilities.
“Our data suggest that application of IFNb to the nasal mucosa might have an important role to play in this setting and argue for urgent clinical assessment of this approach.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Delayed induction of type I and III interferons and nasal epithelial cell permissiveness to SARS-CoV-2 Catherine F Hatton, Rachel A Botting, Maria Emilia Dueñas, Iram J Haq, Bernard Verdon, Benjamin J Thompson, Jarmila Stremenova Spegarova, Florian Gothe, Emily Stephenson, Aaron I Gardner, Sandra Murphy, Jonathan Scott, James P Garnett, Sean Carrie, Rafiqul Hussain, Jonathan Coxhead, Tracey Davey, A John Simpson, Muzlifah Haniffa, Sophie Hambleton, Malcolm Brodlie, Chris Ward, Matthias Trost, Gary Reynolds, Christopher J A Duncan bioRxiv 2021.02.17.431591; doi: https://doi.org/10.1101/2021.02.17.431591, https://www.biorxiv.org/content/10.1101/2021.02.17.431591v1
- Peer reviewed and published scientific report.
Hatton, Catherine F., Rachel A. Botting, Maria Emilia Dueñas, Iram J. Haq, Bernard Verdon, Benjamin J. Thompson, Jarmila Stremenova Spegarova, et al. 2021. “Delayed Induction of Type I and III Interferons Mediates Nasal Epithelial Cell Permissiveness to SARS-CoV-2.” Nature Communications 12 (1). https://doi.org/10.1038/s41467-021-27318-0. https://www.nature.com/articles/s41467-021-27318-0.