Researchers in the United States have conducted a study showing that activation of the complement system is specifically implicated in the pathogenesis of severe coronavirus disease 2019 (COVID-19), rather than being a broader indicator of critical illness.
The complement system is a tightly regulated network of proteins that work together as the first line of host immune defense against invading pathogens.
Now, a team of scientists from Washington University School of Medicine, Marian University, University of Pittsburgh, Yale School of Medicine and Yale New Haven Health System, have identified complement activation as a distinctive immunologic feature of COVID-19.
Hrishikesh Kulkarni of Washington University School of Medicine says complement activation distinguishes those at a higher risk of developing severe disease following infection with the causative agent – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Furthermore, the study indicated that it is enhanced activation of the alternative complement pathway that is implicated in this more severe disease.
The team also pinpointed components of the alternative pathway that are markedly elevated in severe COVID-19, thereby providing specific targets that could be used for risk prediction and drug discovery.
"Our findings may potentially refine the approach for therapeutically targeting the complement system in severe SARS-CoV-2-infection," write the researchers.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The immune components that contribute to severe COVID-19 are not well understood
The morbidity and mortality associated with severe COVID-19 have been attributed to a hyperinflammatory phase and the so-called "cytokine" storm.
However, although high levels of certain cytokines are associated with COVID-19 mortality, their circulating levels are no different from those measured in patients with other viral infections such as influenza.
On the other hand, clinical findings and autopsy reports have indicated that at least in some patients with COVID-19, distinct immunologic responses are responsible for certain coagulopathic events.
"Understanding the underlying mechanisms of these relatively unique aspects of COVID19 is crucial for targeting therapies and may provide insights into the pathogenesis of acute respiratory distress syndrome on a broader scale," write the researchers.
What about the complement system?
The complement system has been implicated in the pathogenesis of severe COVID-19 since early on in the pandemic.
Multiple studies have shown elevated levels of complement activation products such as C5a and sC5b-9 in patients with COVID-19 and deposition of activated complement proteins in injured tissues and organs.
"As a result, these studies have created a precedent for targeting the complement system in multiple ongoing phase II and phase III clinical trials employing complement inhibitors in COVID-19," says Kulkarni and colleagues.
However, many cytokines that have been implicated in COVID-19 are also elevated in other forms of acute infection, including those leading to respiratory failure.
The authors say that most studies to date investigating the complement system's role in COVID-19 have not included control cohorts of patients with other respiratory infections or acute respiratory failure.
Whether complement activation is a unique feature of severe COVID-19 or merely a broader indicator of critical illness, therefore, remains unknown.
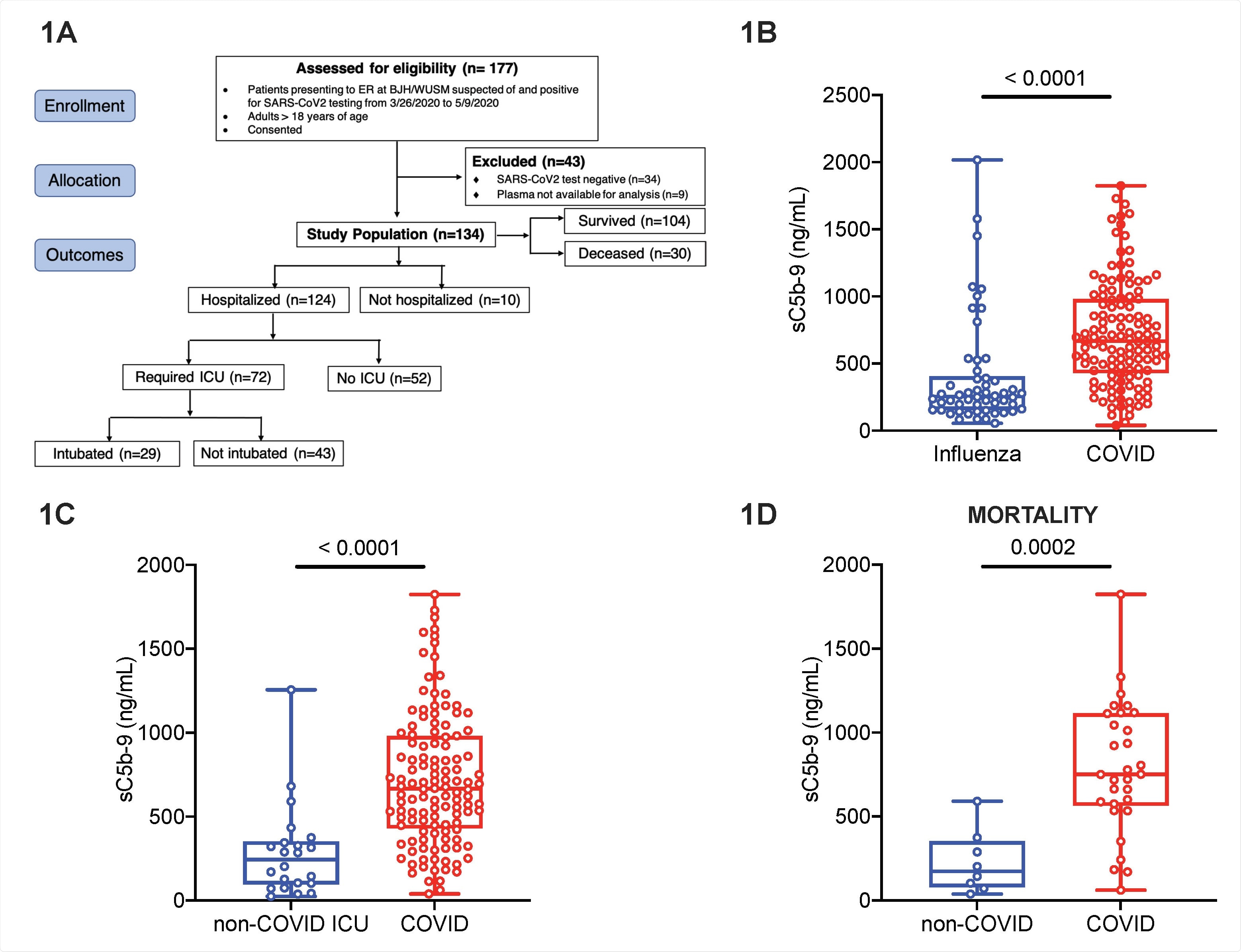
Markers of complement activation are unique to COVID-19 compared to non-COVID-19 respiratory failure. Plasma for determination of circulating markers of complement activation was obtained in patients with COVID-19 and influenza at Barnes-Jewish Hospital (BJH)/Washington University School of Medicine (WUSM). (A) CONSORT flow diagram showing patient enrollment, allocation and outcomes in the COVID-19 cohort. The CONSORT diagram for the influenza and non-COVID acute respiratory failure cohorts are in Figure S1. Box and whiskers plots of differences in sC5b-9 between (B) the influenza (EDFLU) and COVID-19 cohorts, (C) the non-COVID acute respiratory failure (Immunity in Pneumonia and Sepsis, IPS) and the COVID19 cohorts, and (D) restricting the cohorts in Fig.1C to those who died. The center of the box represents the median value, and the length of the box represents the interquartile range. The whiskers represent the minimum and maximum values in each group. Statistical significance is determined using Mann-Whitney U test.
What did the current study find?
The team investigated complement activation in blood samples from 134 COVID-19 patients presenting at emergency departments, 54 patients hospitalized with influenza, and 22 intensive care unit (ICU) patients with non-COVID-19 acute respiratory failure.
The researchers found that circulating markers of complement activation such as sC5b-9 were higher in patients with COVID-19 than in patients with influenza or non-COVID-19 respiratory failure.
These markers of complement activation helped distinguish COVID-19 patients who were at a higher risk of worse outcomes, such as requiring ICU admission or invasive mechanical ventilation.
Furthermore, the results pointed to enhanced activation of the alternative complement pathway in patients with severe COVID-19 that was also responsible for the worse outcomes in this group.
In addition, components of the alternative pathway were associated with markers of endothelial injury such as angiopoietin-2 and markers of hypercoagulability such as thrombomodulin and von Willebrand factor.
The researchers say these markers are also the clinico-physiological hallmarks of severe COVID-19 vasculopathy.
What should be the next step?
"The next step would be to understand the mechanistic basis for this interaction and to evaluate whether interrupting alternative pathway-mediated activation could mitigate this vicious cycle that perpetuates tissue injury, at least in a subset of patients with severe COVID-19 who have this phenotype," says Kulkarni and colleagues.
"Much work remains to be done to better understand how and when to target the complement cascade, with the goal of mitigating disease severity due to SARS-CoV-2," concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Kulkarni HS, et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. bioRxiv 2021. doi: https://doi.org/10.1101/2021.02.22.432177, https://www.biorxiv.org/content/10.1101/2021.02.22.432177v1
- Peer reviewed and published scientific report.
Ma, Lina, Sanjaya K. Sahu, Marlene Cano, Vasanthan Kuppuswamy, Jamal Bajwa, Ja’Nia McPhatter, Alexander Pine, et al. 2021. “Increased Complement Activation Is a Distinctive Feature of Severe SARS-CoV-2 Infection.” Science Immunology 6 (59): eabh2259. https://doi.org/10.1126/sciimmunol.abh2259. https://www.science.org/doi/10.1126/sciimmunol.abh2259.