As newly developed vaccines against coronavirus disease 2019 (COVID-19) are being rolled out worldwide, several regions have seen the rise of vaccine hesitancy or unwillingness. This is to a large extent fuelled by fears about the potentially serious adverse effects of the vaccines.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Vaccine-associated adverse effects
The first two vaccines to enter the arena were the BNT162b2 (Pfizer/BioNTech) and mRNA-(Moderna) COVID-19 vaccines. Both received Emergency Use Authorizations by the Food and Drug Administration (FDA), and the phase III trials showed that they were effective and safe, with efficacies hovering around 95%.
Local adverse effects reported in phase III trials included pain, redness, and/or swelling at the site of injection, with the first being by far the most common. Systemic reactions included headache, fatigue, muscle pain, joint pain, fever with chills, and nausea or vomiting.
Real-time AI-driven analysis
The need to evaluate the real-time safety and efficacy of these vaccines in the broad world population drove the current study. The method they chose was the analysis of patient information derived from electronic health record (EHR) systems.
Earlier, these researchers had set up an algorithm to select and categorize patient data from HER, in order to obtain a cohort of vaccinated patients tested for COVID-19 by polymerase chain reaction (PCR) with clinically-matched unvaccinated controls.
This was used in the current study to acquire information on real-world vaccine safety in over 31,000 patients who were vaccinated through the Mayo Clinic and its associated centers.
The differences in evaluating vaccine-associated adverse effects in and outside a clinical trial vary in the number and types of adverse effects reported. In the first setting, volunteers were encouraged to report any adverse effect, while in the second, only those effects that are tolerably severe are likely to be reported to the physician, as the patient must take an additional effort to book a consultation in such a case.
The study matches the vaccine recipients to matched controls, helping to adjust the adverse effects reported in terms of context and other factors.
No increase in clinic visits
The study found that there was no unexpected spike in clinic or physician visits following vaccination at 7, 14 or 21 days from the first or second dose. At seven days, about 20% of vaccinated individuals had been seen at least once by a physician, comparable to unvaccinated individuals. The same applies at later time points.
With the second dose, too, about 14% to 16% had been seen by a physician, in both cohorts, within 7 days, and the same pattern was repeated at 14 and 21 days. These patterns indicate that the vaccines are tolerable in the general population.
No increase in adverse event reporting
The rates at which vaccine-associated adverse effects were reported did not rise above the occurrence of such symptoms in unvaccinated controls. They were found to be much lower than reported during clinical trials, as expected. In real life, those reported to healthcare following a vaccine dose are adverse effects that are either severe or persistent enough to prompt a return to the clinic or other medical center.
The symptoms most often reported included headache, muscle pain, joint pain and tiredness, and were equally reported within a week of the first dose, as well as of the second dose (whether this was actually taken or assigned).
Serious adverse effects such as anaphylaxis and facial palsy were also comparably reported in this time period for both cohorts. Some symptoms, like fever, diarrhea and lymph node enlargement, were less frequent in the vaccinated cohort, in fact, at all three time points.
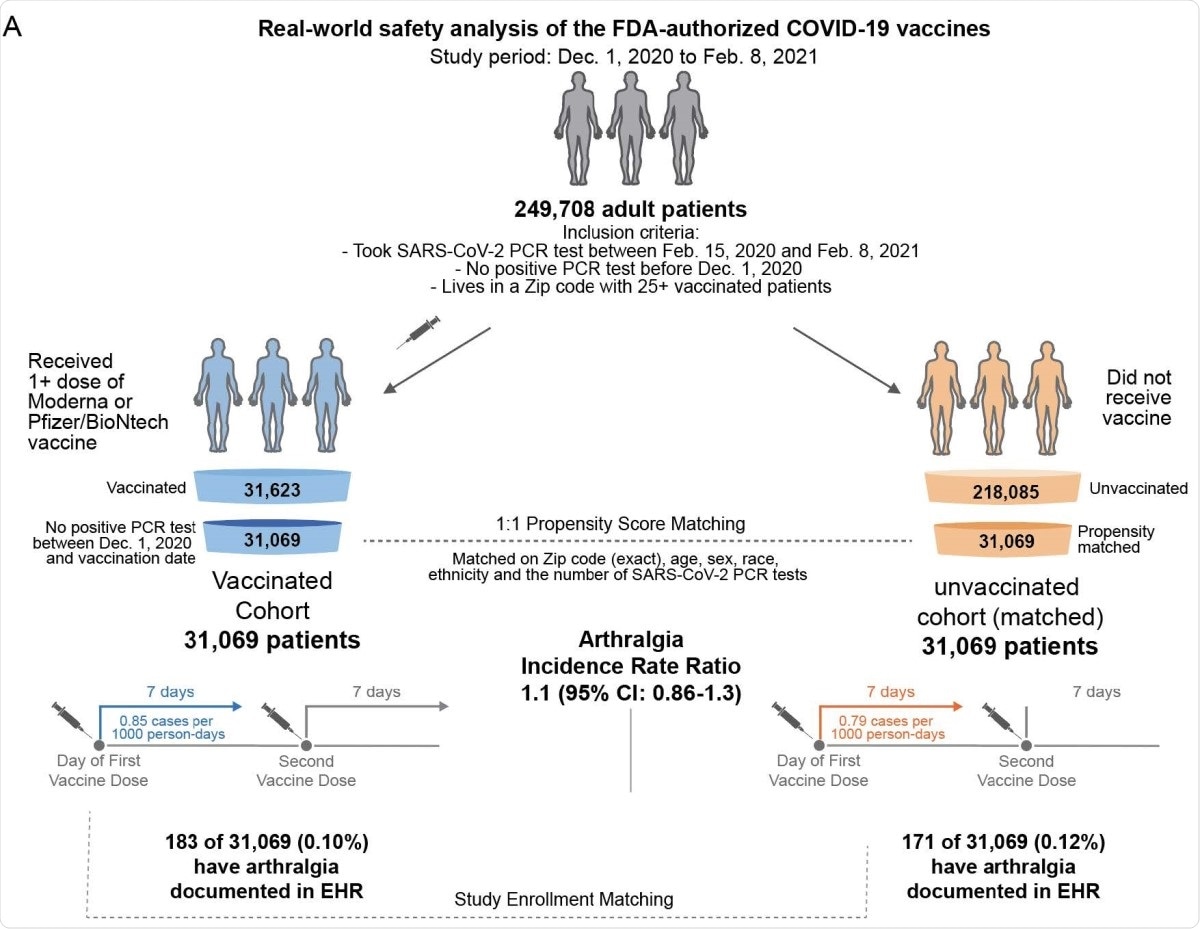
Schematic illustration of participant selection and study design. The vaccinated cohort is composed 31,069 individuals from the Mayo Clinic and associated health systems who received at least one dose of BNT162b2 (Pfizer/BioNTech) or mRNA-1273 (Moderna) between December 1, 2020 and February 8, 2021 and did not test positive for SARS-CoV-2 prior to their first vaccination. A control cohort of unvaccinated individuals was generated via 1:1 propensity score matching, yielding 31,069 individuals with similar distributions of age, sex, race, ethnicity, residential location, and number of prior SARS-CoV-2 PCR tests in the past year. For each cohort, the incidence rates of several adverse effects (e.g., arthralgia) were calculated for the seven days following the first dose and, separately, for the seven days following the second dose. For a given adverse effect, the incidence rate ratio (and the corresponding 95% CI) was calculated to determine whether one cohort was more likely to experience the event than the other. Incidence rates and incidence rate ratios were also calculated for the 14 and 21 days following each vaccine dose.
What are the implications?
The low rates of reported symptoms are attributed to their mildness, in most cases, as well as to the fact that healthcare practitioners are likely to make up a large part of the vaccinated group due to their prioritization during the vaccination campaign. As such, being familiar with the expected adverse effects, they are less likely to report them unless unexpectedly or intolerably severe.
This does not apply to serious issues, including anaphylaxis, which are more likely to be documented because of the need for emergency care. These were also not more frequently reported in the study.
Our method should identify the symptoms and phenotypes that represent the most serious threats to vaccine safety and tolerability of practical significance. Indeed, this is the central reason why our analysis should be viewed as complementary to the data which has been obtained in the more controlled setting of clinical trials.”
The researchers conclude that their findings support the safety and tolerability of both the Pfizer and Moderna vaccines, providing a better foundation for their rapid and broad-based distribution.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
McMurry, R. et al. (2021). Real-time analysis of a mass vaccination effort via an Artificial Intelligence platform confirms the safety of FDA-authorized COVID-19 vaccines. medRxiv preprint doi: https://doi.org/10.1101/2021.02.20.21252134, https://www.medrxiv.org/content/10.1101/2021.02.20.21252134v1
- Peer reviewed and published scientific report.
McMurry, Reid, Patrick Lenehan, Samir Awasthi, Eli Silvert, Arjun Puranik, Colin Pawlowski, A.J. Venkatakrishnan, et al. 2021. “Real-Time Analysis of a Mass Vaccination Effort Confirms the Safety of FDA-Authorized MRNA COVID-19 Vaccines.” Med 2 (8): 965-978.e5. https://doi.org/10.1016/j.medj.2021.06.006.https://www.cell.com/med/fulltext/S2666-6340(21)00237-3.