The first instance of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak in Austria was observed in the Ski resort Ischgl in early March 2020. Later, the virus spread across Austria and other countries in Northern Europe.
Researchers conducted a cross-sectional study to gauge the seroprevalence of SARS-CoV-2 in the population, as measured in the blood serum. They found that the seroprevalence in the adult population had increased to 45% by the end of April.
Subsequently, researchers sought to answer a couple of important questions, namely, how long does immunity last and what was the effect of this heightened immunity on virus transmission. To this end, a follow-up study (Ischgl - 2) was conducted in the first week of November 2020.
A new research paper released on the medRxiv* preprint server documents the findings of this follow-up study. Researchers document that T-cell and antibody responses could still be observed in most individuals, six and a half months after the first study and almost eight months after the first wave in Ischgl. They also found that the number of new COVID-19 cases reported from May to December was very low.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
In terms of the sample size, the original study included 1,473 individuals, including 214 children. In the follow-up study, only 801 individuals from the original group could be included. Some individuals had to be excluded owing to inconsistent age, lack of questionnaire, or lack of blood samples. A total of 91 new individuals entered the follow-up study, including 12 children. Akismed, a web-based eCRF system, aided in the data collection exercise.
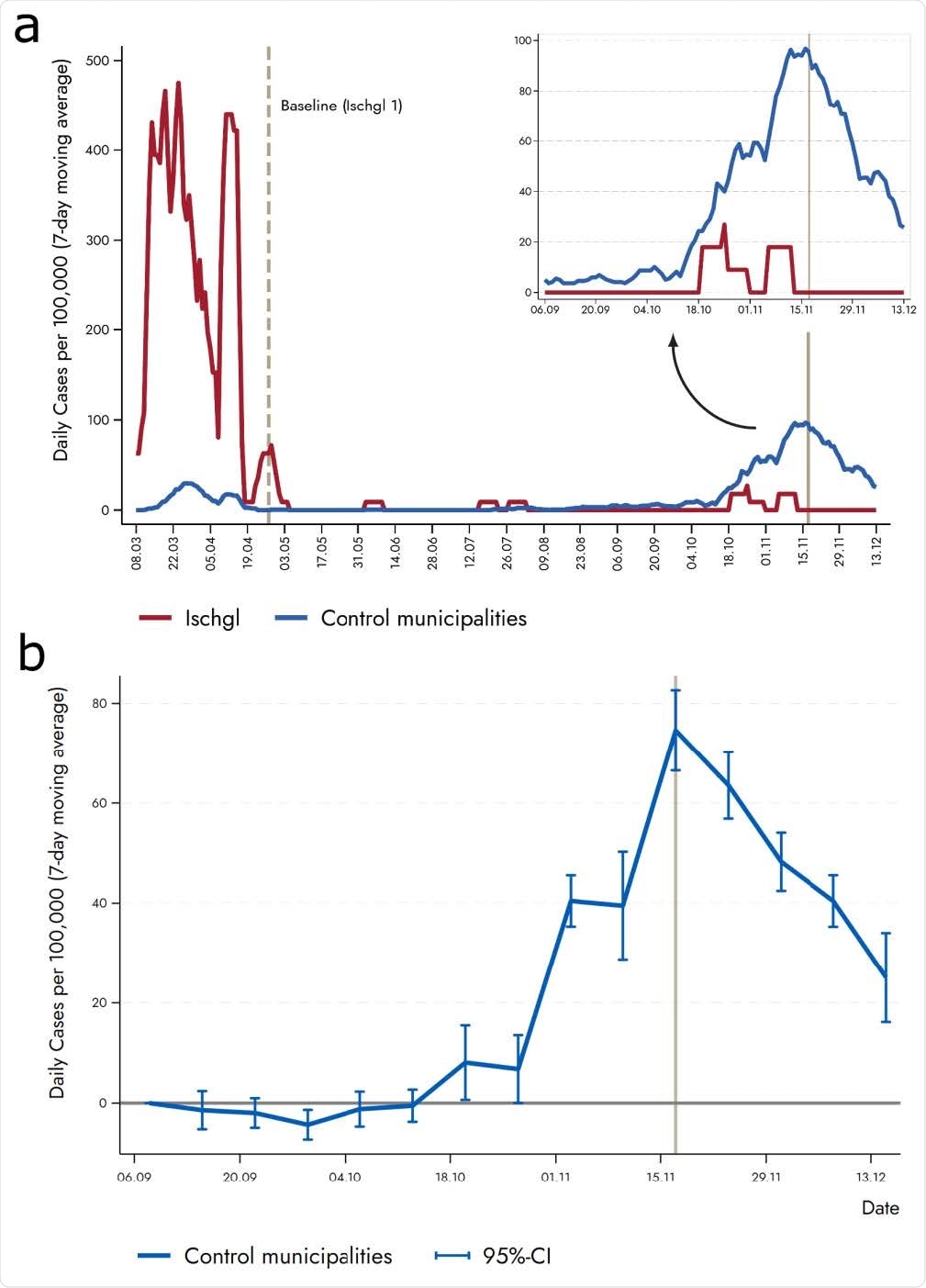
Lower incidence of new infections in Ischgl compared to low-prevalence villages. (a) Vertical solid line represents the second countrywide lockdown in autumn 2020, which took place in November 17 (a first and lighter lockdown took place on November 2). The control municipalities are Eben am Achensee, Ellmau, Fiss, Gerlos, Lermoos, Leutasch, Mayrhofen, Nauders, Neustift im Stubaital, Seefeld in Tirol, Serfaus, Sölden, and Tux. (b) The figure displays results from regression equation (1). The plotted coefficients represent the weekly difference in the 7-day moving average of new cases between Ischgl and the control municipalities relative to the reference period (2nd week of September). The coefficient for each week is shown together with a 95%-confidence interval. The vertical solid line shows the second (strict) lockdown in Austria.
The methodology involved the analysis of EDTA-plasma using four immunoassays. ImmunomatTM, a fully automated 4-plate benchtop instrument, was used to screen the blood samples for anti-SARS-CoV-2-S1 protein Immunoglobulin A (IgA) and Immunoglobulin G (IgG) positivity using anti-SARS-CoV-2-IgA and –IgG ELISA, respectively.
The threshold for considering the samples to be positive was set at 1.1 and the interpretation of these results with respect to the obtained optical density values was guided by the information provided by the manufacturer.
Values in the range of 0.8 - 1.1 in the IgG ELISA were considered positive and such borderline values were considered negative for the IgA ELISA. Researchers also tested each sample for the anti-SARS-CoV-2-N-protein IgG (anti-N IgG) with the Abbott SARS-CoV-2 immunoassay. The anti-N IgG was considered positive for the relative light unit (RLU) values greater than 1.4.
Additional quantification of the anti-N IgG was performed using the ElecsysAnti-SARS-CoV-2, developed by Roche Diagnostics. The Roche assay provides a cut-off index where values greater than 1.0 are considered positive.
Lastly, a neutralizing antibody assay was also used to determine titers of SARS-CoV-2 neutralizing antibodies using vesicular stomatitis virus (VSV) pseudotyped with SARS-CoV-2 spike protein.
The next steps were to define seroprevalence and serostatus of the samples, determine the avidity of SARS-CoV-2-specific anti-S igG, and analysis of SARS-CoV-2-specific T cell responses.
Additional data on demographics was required. Means, medians, standard deviations, and interquartile ranges were calculated for continuous variables.
For categorical variables, numbers (percentages) were calculated. Cross-sectional seroprevalence was calculated in November for the whole sample.
For individuals whose data was available both for the baseline and follow-up studies, the seroprevalence was calculated. This concluded the data gathering phase, following which researchers performed statistical analysis using STATA 16.1 and GraphPad Prism version 8.2.0 and 9.
Some of the statistical techniques used by the researchers were ANOVA, non-parametric methods (Mann-Whitney U-test, Kruskal-Wallis test), Student’s t-test for paired samples, Wilcoxon signed-rank test, regression analysis, etc.
Researchers found that the levels of antibodies declined in the first 7-8 months after infection. However, the binding capacity of antibodies increased and/or T cell responses could still be detected in all but one individual.
The open question is how long does the immunity last for SARS-CoV-2? SARS-Cov-2 causes a more serious systemic infection than other human coronaviruses that cause the common cold. In the latter case, immunity is known to last 1-2 years but, in this case, the immunity could last longer.
This idea is supported by prior studies that document that the immune response to SARS-CoV-1 remains detectable for longer than 17 years.
The conclusion of this study that immunity may last for at least eight months is in line with the fact that reinfections are rare in the world (zero in Ischgl).
Researchers also documented that the incidence of SARS-CoV-2 in Ischgl was significantly lower than other comparable municipalities in Austria during the second wave in November.
The findings suggest that a 40-45% level of seropositivity may be sufficient to stop the virus's spread with some social distancing measures in place.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Follow-up study in the ski-resort Ischgl: Antibody and T cell responses to SARS-CoV-2 persisted for up to 8 months after infection and transmission of virus was low even during the second infection wave in Austria, Wegene Borena, Zoltán Bánki, Katie Bates, Hannes Winner, Lydia Riepler, Annika Rössler, Lisa Pipperger, Igor Theurl, Barbara Falkensammer, Hanno Ulmer, Andreas Walser, Daniel Pichler, Matthias Baumgartner, Sebastian Schönherr, Lukas Forer, Ludwig Knabl, Reinhard Würzner, Dorothee von Laer, Jörg Paetzold, Janine Kimpel, medRxiv, 2021.02.19.21252089; doi: https://doi.org/10.1101/2021.02.19.21252089, https://www.medrxiv.org/content/10.1101/2021.02.19.21252089v1
- Peer reviewed and published scientific report.
Borena, Wegene, Zoltán Bánki, Katie Bates, Hannes Winner, Lydia Riepler, Annika Rössler, Lisa Pipperger, et al. 2021. “Persistence of Immunity to SARS-CoV-2 over Time in the Ski Resort Ischgl.” EBioMedicine 70 (August). https://doi.org/10.1016/j.ebiom.2021.103534. https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(21)00327-3/fulltext.