Caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus disease 2019 (COVID-19) pandemic continues to spread across the globe. The rise to dominance of several new variants of concern, some with observed heightened transmissibility, has contributed to a resurgence in cases in many parts of the world – particularly in the U.S., the U.K., Brazil, and South Africa. To date, over 107 million cases of COVID-19 have been confirmed worldwide, along with over 2.35 million deaths.
Since the virus was first detected in Wuhan, China, in December 2019, the global scientific community has been mounting an unprecedented research effort to better understand SARS-CoV-2 – from its transmission dynamics, to its complex mechanisms of infection, to its pathogenicity.
Scientists the world over continue to study the virus and attempt to develop safe and effective prophylactics and therapeutics – from vaccines to antiviral drugs – to prevent its spread and to mitigate disease severity in those it reaches.
In terms of vaccine development, as one of the worst public health crises in living memory, the COVID-19 pandemic has stimulated an enormous feat of nanotechnological innovation in the space of a year. Out of the social and economic chaos of 2020, stepped the messenger ribonucleic acid (mRNA) vaccine models.
Most notably, the BNT162b2 candidate – also known as the 'BioNTech-Pfizer vaccine' – which was the first to receive a national regulatory body's approval and to be administered to members of a population.
Evonik strengthens strategic partnership with BioNTech on COVID-19 vaccine. Image Credit: LookerStudio / Shutterstock
BioNTech-Pfizer model
Following the onset of the COVID-19 pandemic in late-2019, the German biotechnology firm, BioNTech, teamed up with a pharmaceutical giant, Pfizer, to develop and trial a vaccine that would prevent SARS-CoV-2 infection.
By early-November 2020, the BioNTech-Pfizer partnership announced positive outcomes for their third and final phase of clinical trials, which involved human volunteers. In these assessments, the vaccine yielded an efficacy of 95%. This is significantly higher than the World Health Organization's (WHO) recommended target of 70%.
Through an emergency measures process called 'rolling review,' the U.K.'s regulatory body, the Medicines and Healthcare products Regulatory Agency (MHRA), had been assessing the candidate's clinical trial data as and when it was being released to speed up the process. Having scrutinized the trial data in consultation with the U.K.'s expert scientific advisory board, SAGE, the MHRA had officially approved the vaccine's use by December 2020.
Shortly after, the U.S. Food and Drug Administration (FDA) authorized emergency approval of the candidate to vulnerable individuals more susceptible to severe disease and to frontline healthcare workers at high risk of viral exposure.
To date, the Pfizer vaccine has been approved and administered in several countries worldwide, from Canada to Israel to Belgium to Costa Rica.
This radically new model of vaccine has hit the market in record time. In what would usually take as much as a decade, the BioNTech-Pfizer vaccine, as well as its rival mRNA candidate, the 'Moderna vaccine', were developed, trialed, approved, and rolled out in the space of a year.
But what exactly are mRNA vaccines? And how do they work?
The mRNA vaccine model
SARS-CoV-2 infects host cells through the angiotensin-converting enzyme 2 (ACE2), a host cell receptor that is abundantly expressed in human epithelial tissue. Epithelia is especially present in the human airway, and it is by this means that SARS-CoV-2 is a predominantly respiratory infection.
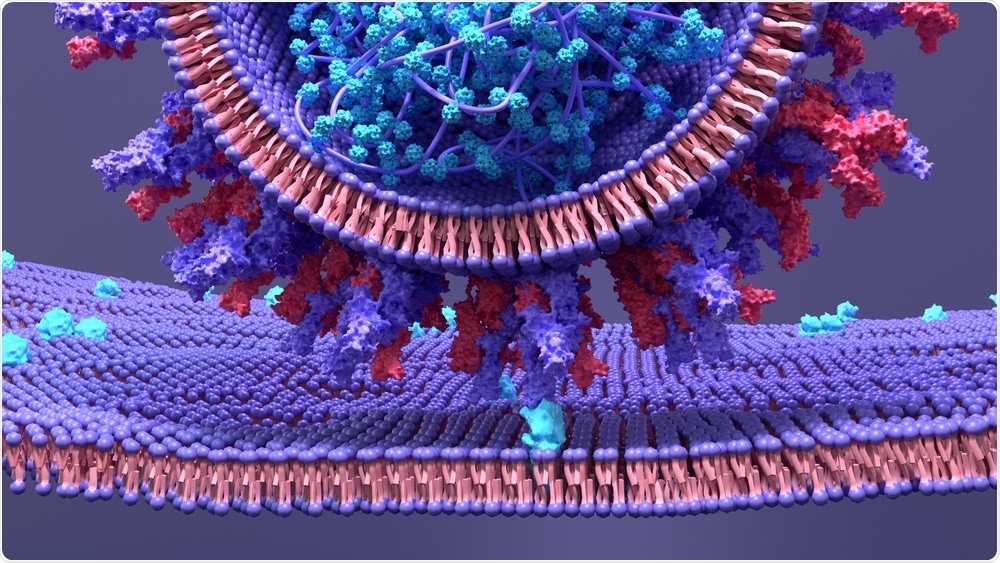
SARS-CoV-2 binds to the ACE2 receptor of host cells to initiate infection. Image Credit: Design_Cells / Shutterstock
The SARS-CoV-2 microbe’s surface is adorned with spike proteins (or S proteins), which latch onto the ACE2 receptor and instigate host-virion membrane fusion. From here, the virus hijacks the host cell's metabolic processes and instigates viral replication.
While traditional vaccines typically inject an attenuated or inactivated version of the virus to elicit a robust immune response, the BNT162b1 vaccine is part of a new class of messenger ribonucleic acid (mRNA) vaccine that injects a part of SARS-CoV-2's genetic material within a surrogate lipid nanoparticle (LNP).
The mRNA is a piece of the virus’s genetic code that contains the message for building the spike protein. This then stimulates the host's cells to produce spike protein antigens, which, in turn, induces their immune system to produce neutralizing antibodies against it. These anti-SARS-CoV-2 antibodies persist as part of the host’s immune system for an extended period of time, conferring protection from infection – or from severe disease at the least – if they are exposed to the virus out in the community.
The vaccine requires two shots (a primer and a booster) at least 21 days apart for optimal efficacy.
Evonik teams up with BioNTech
Given the enormous scale of vaccination efforts all over the world, effective supply chains for the vaccines themselves will be essential in turning the tide of the pandemic. The timely supply of their constituent parts is a crucial dimension of this.
Evonik, a specialty chemicals company in Essen, Germany, is investing in the short-term expansion of its specialty lipids production, which are an integral ingredient in mRNA-based COVID-19 vaccines.
Commercial lipid quantities are to be produced at Evonik's Hanau and Dossenheim sites in Germany as early as the second half of 2021 as part of a strategic partnership with BioNTech. In providing this essential service, Evonik is making an important contribution to increasing the supply security of COVID-19 vaccine.
The pandemic requires decisive action. We are therefore doing everything possible to supply our partners with the critical lipids they need. At the same time, we are expanding our production capacity and competencies along the entire value chain."
Christian Kullmann, chairman of Evonik's executive board.
As lipids are a fundamental ingredient of mRNA-based vaccines to enable the delivery of mRNA to the human cells, only an increase in lipid supply can boost the volume of vaccines currently being produced. This move thus marks an expansion of the strategic partnership between Evonik and the vaccine manufacturer BioNTech to aid in the global vaccination effort.
Evonik has been a contract development and manufacturing organization (CDMO) leader for advanced drug delivery for many decades, supporting pharmaceutical companies worldwide in the development and production of complex parenteral drug products that require formulation technologies such as lipid nanoparticles.
Evonik already supplies one of the most important lipids for mRNA vaccines in pharmaceutical quality to multiple customers: A non-animal derived cholesterol under the brand name PhytoChol®. In Hanau and Dossenheim, Evonik will soon produce two other specialty lipids needed to manufacture the COVID-19 vaccine.
The company is one of the few integrated development and manufacturing partners for cell and gene therapies and has been actively involved in various mRNA-based vaccine projects for COVID-19.
With our partnership with BioNTech, we are systematically expanding our leading position as an integrated development partner in cell and gene therapies. Evonik's portfolio includes pharmaceutical excipients such as lipids, as well as CDMO services for the formulation development, GMP manufacturing and aseptic filling of complex parenteral drug products."
Thomas Riermeier, head of Evonik's Health Care business line.