In the light of the enormous number of cases of coronavirus disease 2019 (COVID-19) that have been documented so far in the ongoing pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is a great need to understand how this infection affects the immune system long-term.
A new study by researchers at the University of California, San Francisco, discusses the different aspects of long-term immune responses in a wide sample of patients, as well as how they vary in clinical severity and sequelae.
The majority of specific cellular immune responses to SARS-CoV-2 infection comprise CD4 and CD8 T cell responses. The current study aims to explore the magnitude of such changes, the presence of soluble inflammatory markers, and of neutralizing and total antibody titers over time, up to 9 months post-infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The team has released their findings as a preprint on the medRxiv* server.
Study details
The study group included 70 individuals in whom the infection had been confirmed by polymerase chain reaction (PCR), but had an illness of varying severity. All were from northern California, as part of the Long-term Impact of Infection with Novel Coronavirus (LIINC) cohort.
Patients in the study were mostly enrolled within 40 days of symptom onset or had been followed up longitudinally over time. The researchers also examined peripheral blood mononuclear cells (PMBCs), plasma and serum samples for soluble inflammatory markers and antibodies.
The participants were equally matched for males and females. A quarter of them had been hospitalized, and most of these (except for two) required oxygen supplementation. Intensive care was required by 14%.
More males than females were hospitalized, as expected, at ~40% vs 10% overall, and 100% of those who required intensive care. About 60% of hospitalized patients were Latinx in origin.
Almost a fifth had a history of lung diseases, such as asthma or chronic obstructive pulmonary disease (COPD).
Almost all the participants had symptoms related to COVID-19 when or soon after they were diagnosed, including cough, shortness of breath, fever, anosmia and ageusia, brain fog, tiredness, neuropathies, headache and loss of concentration. These were reported in over 70% of patients.
About 46% had one or more such symptoms at a median of 53 days after symptom onset or first positive PCR test. About 36% still had symptoms at four months.
Females tended to be more likely to have lingering symptoms at both time points, though they were less likely to be hospitalized.
Stable T cell responses
The study shows that, as earlier reported, the magnitude of the initial T cell response depends on the severity of the illness. However, following recovery, the cellular immune response remains stable for up to eight months.
The frequency of spike (S)- or nucleocapsid (N)-specific CD4 and CD8 T cells in peripheral blood in patients exposed to the virus, and therefore to the S and N proteins, was found to be elevated in almost all cases.
About a quarter of these specific IFNγ+ CD8 cells and 60% of IFNγ+ CD4 T cells were found to produce TNFα. The T cell frequency remained stable over the study period, except that N-specific IFNγ+ CD4 T cells showed a slight decline.
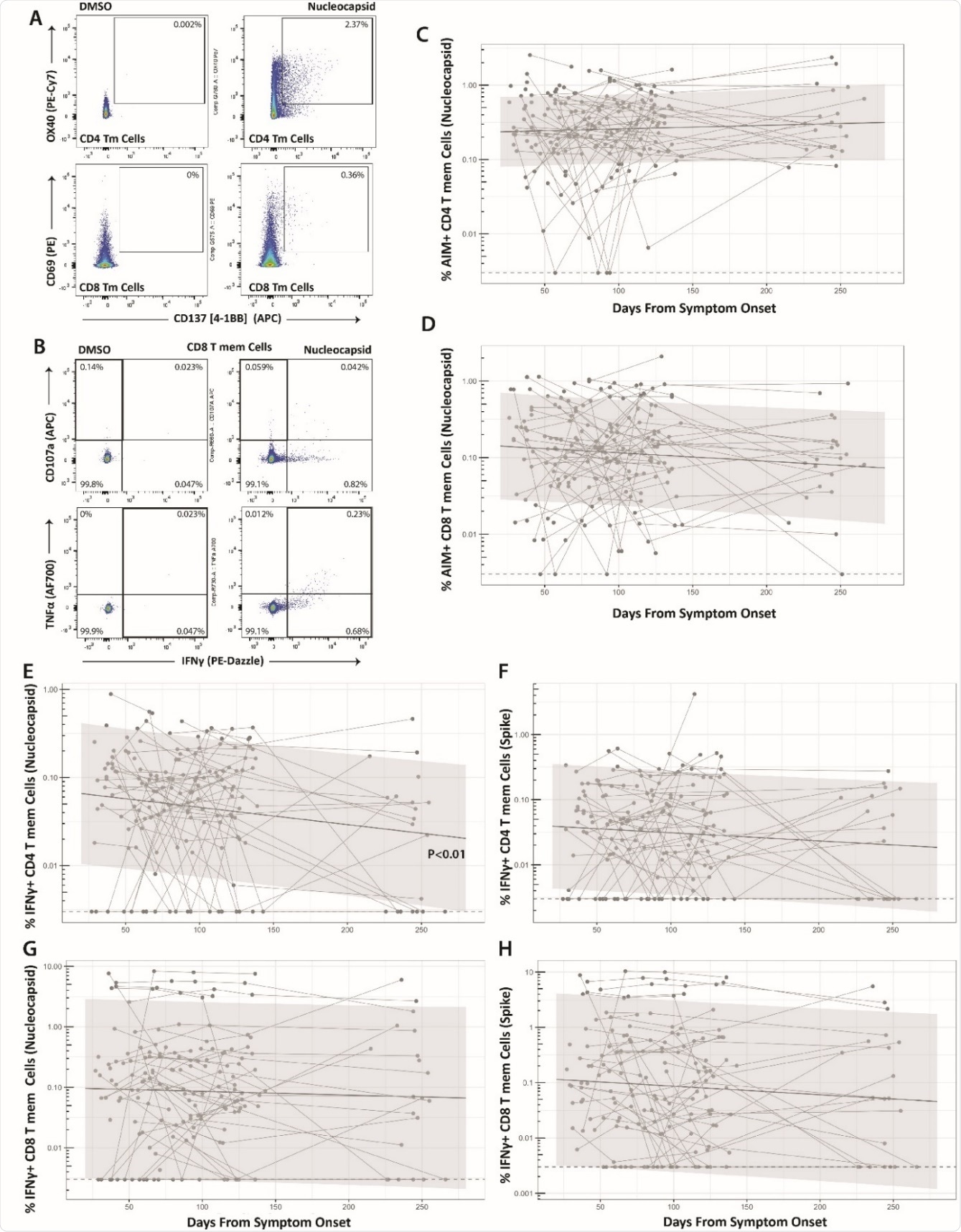
Long-term durability of SARS-CoV-2-specific CD4 and CD8 cell responses. Gating strategy for identifying SARSCoV-2-specific memory T cell responses in the activation-induced marker (AIM) (A) and intracellular cytokine staining (ICS) (B) assays. Percentage of AIM+ N-specific CD4+ (C) and CD8+ (D) T cell responses over time (S-specific responses were similar and are shown in Supplementary Figure 3). Percentage of IFNγ+ Nucleocapsid (N)- or Spike (S)-specific CD4 (E-F) and CD8 (G-H) T cell responses over time. Points and connecting lines represent raw data for each individual. Solid line and shaded region represent the median model prediction and 95% prediction interval from linear mixed effects modeling including individual effects. Dashed lines represent assay limits of detection. T mem = T memory cells.
Early acute responses in hospitalized patients
Again, the early T cell response in patients who required hospitalization because of acute infection was made up of N- and S-specific IFNγ+CD4 T cells. The frequencies of both were higher in these patients, including those who required intensive care. These higher responses were observed throughout the follow-up period.
Patients who were over 50 years had markedly higher S-specific IFNγ+ CD4+ T cell percentages at the later sampling time point, though not at the initial sampling point. This did not preclude a general decline in the percentage of these cells in hospitalized COVID-19 patients.
Asian patients showed an initially higher frequency of S-specific IFNγ+ CD8+ T cells relative to white non-Latinx participants, but this was not observed at subsequent sampling.
However, with patients who have a history of chronic lung disease, the long-term S-specific IFNγ+ CD4+ and IFNγ+ CD8+ T cell responses were found to be higher throughout. This stable high response was not correlated with the severity of the initial illness or the patient’s age.
If the COVID-19 patient with chronic lung disease was hospitalized, the researchers found lower N-specific IFNγ+ CD8+ T cells at the last sampling point but higher frequencies in those who were not hospitalized.
When adjusted for age, the initially higher N and S-specific IFNγ+ CD4+ T cell responses in hospitalized patients relative to non-hospitalized, as well as the increased S-specific IFNγ+ CD4+ T cell response at the last time point, remained significant.
Specific antibodies and CD4 T cells
The levels of anti-S, anti-N and anti-RBD antibodies stabilized with time, with higher titers in the hospitalized patients, and, at the later sampling time point, higher anti-N antibodies among those aged 50 years or more. The difference in antibody titers could be driven by disease severity, therefore, in the acute phase of the disease.
The titers of neutralizing antibodies showed a robust correlation with the specific anti-S and anti-N CD4 T cell responses only.
Soluble inflammatory markers
The levels of inflammatory molecules such as IL-6, IL-10, IP-10, and D-Dimer remained stable over time, but the first two were somewhat lower in hospitalized patients. However, IL-10 remained a little higher in hospitalized patients across the follow-up period and in those with persistent or long-haul symptoms.
6, IL-10, and IP-10 were strongly positively correlated with each other but not with D-Dimer, sCD14 or sCD163.”
What are the implications?
The study shows that adaptive immunity is somewhat stable over eight months in patients who have been infected with SARS-CoV-2 at various ages and with different clinical severities. This does not reflect the strength of protective immunity, but the durable nature is promising.
Long-term immune cell responses appeared to be unrelated to sex differences in this study, despite the reported differences in early immune responses and immune activation during acute infection.
The absence of discernible waning in specific or adaptive immunity during early recovery with age is an encouraging sign, but older patients had a higher CD4 T cell response at the later sampling. CD8 T cells did not show this difference with age.
Longer follow-up may be necessary to unravel other differences in memory T cell responses with age. Equally, it may be that the frequencies of different T cells remain stable, but immune responses become less coordinated.
Hospitalization was associated with higher frequencies of memory CD4 T cells earlier in recovery, but not after four months. However, patients who had required intensive care did have higher CD4 T cell responses, mostly against the S, N and RBD.
CD8 T cells were persistently higher in COVID-19 patients with pre-existing lung disease, independent of age or hospitalization. However, those in this category who had been hospitalized with COVID-19 had lower CD8 T cell responses.
Finally, the study failed to show significant differences in the humoral or specific cellular responses in patients with differing long-term outcomes, vs those who recovered rapidly without such sequelae. As earlier reports indicate, the researchers observed that women were more likely to have persistent symptoms at the first convalescent visit, at almost 50%.
However, despite the emergence of clear symptom profiles, the underlying immunologic response did not show much difference between the patients who recovered and those who did not. This type of understanding of the multiple factors underlying long-haul COVID-19 may require larger studies with clearly defined different groups of participants.
The strong correlation between neutralizing antibodies and S- and N-specific CD4+ T cells indicates that follicular helper T cell responses, occurring within the lymph nodes in SARS-CoV-2 clearance. Further research is warranted to understand how humoral and cellular responses act in synergy in different population groups to overcome the infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Peluso, M. J. et al. (2021). Long-Term SARS-CoV-2-Specific Immune and Inflammatory Responses Across a Clinically Diverse Cohort of Individuals Recovering from COVID-19. medRxiv preprint. doi: https://doi.org/10.1101/2021.02.26.21252308, https://www.medrxiv.org/content/10.1101/2021.02.26.21252308v1
- Peer reviewed and published scientific report.
Peluso, Michael J., Amelia N. Deitchman, Leonel Torres, Nikita S. Iyer, Sadie E. Munter, Christopher C. Nixon, Joanna Donatelli, et al. 2021. “Long-Term SARS-CoV-2-Specific Immune and Inflammatory Responses in Individuals Recovering from COVID-19 with and without Post-Acute Symptoms.” Cell Reports 36 (6). https://doi.org/10.1016/j.celrep.2021.109518. https://www.cell.com/cell-reports/fulltext/S2211-1247(21)00948-7.