Viruses evolve under increased pressure, and new research from The Rockefeller University and University of Massachusetts Medical School in the U.S. suggests our antibodies can as well.
The team found antibodies targeting the receptor-binding domain (RBD) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), can mutate to have more neutralizing ability and more resilience to viral mutation. The increased antibody diversity could further protect against the new coronavirus variants circulating worldwide and future threats during the pandemic.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The number of global cases and deaths continues to rise. To date, over 117.5 million cases have been confirmed worldwide, resulting in over 2.6 million deaths.
The researchers write:
Antibody maturation may be especially important as SARS-CoV-2 diversifies and adapts to the range of human antibodies in vaccinated and previously infected individuals. Moreover, a diverse set of broadly neutralizing SARS-CoV-2 spike-elicited antibodies that exhibit some activity against divergent sarbecoviruses may mitigate the threat posed by this group of pandemic-threat agents.”
The study “Development of potency, breadth and resilience to viral escape mutations in SARS-CoV-2 neutralizing antibodies” is available as a preprint on the bioRxiv* server, while the article undergoes peer review.
Antibody mutations are beneficial against SARS-CoV-2
The researchers investigated the characteristics of six antibody lineages and the effect of somatic mutations. They collected samples from patients ranging from mild to moderately severe COVID-19 infection at 1.3 months or 6.2 months. To test neutralizing potency, the researchers exposed the antibodies to HIV-1 SARS-CoV-2 pseudotypes with single mutations that have been associated with helping the coronavirus evade the immune system.
We show that somatic mutations acquired in the months after infection endow some SARS CoV-2 RBD-specific antibodies with greater neutralization potency and breadth. Most importantly, the acquisition of somatic mutations provides some antibodies with resilience to viral mutations that would otherwise enable SARS-CoV-2 to escape their neutralizing effects.”
Mutations in neutralizing antibodies targeting SARS-CoV-2 included greater potency, which was observed in antibodies isolated at 6.2 months than those at 1.3 months.
A benefit to diversity in B cell memory cells is the ability to detect foreign invaders that are closely related to other pathogens they’ve encountered. The researchers investigated this in mutating antibodies as it could help with recognizing new coronavirus variants that differ by a few mutations.
The researchers found that mutational changes in resistance were observed in 6.2 months of antibodies compared to the antibodies found after 1.3 months. This included limiting chances for viral escape and resilience of neutralization activity.
The neutralizing potency remained in matured antibodies such as C549, C099, and C080 when exposed to the spike protein RBD and when slightly altered through substitutions. Matured antibodies from the C098/99 lineage also showed resistance and neutralizing ability towards RBGs with two or more mutations.
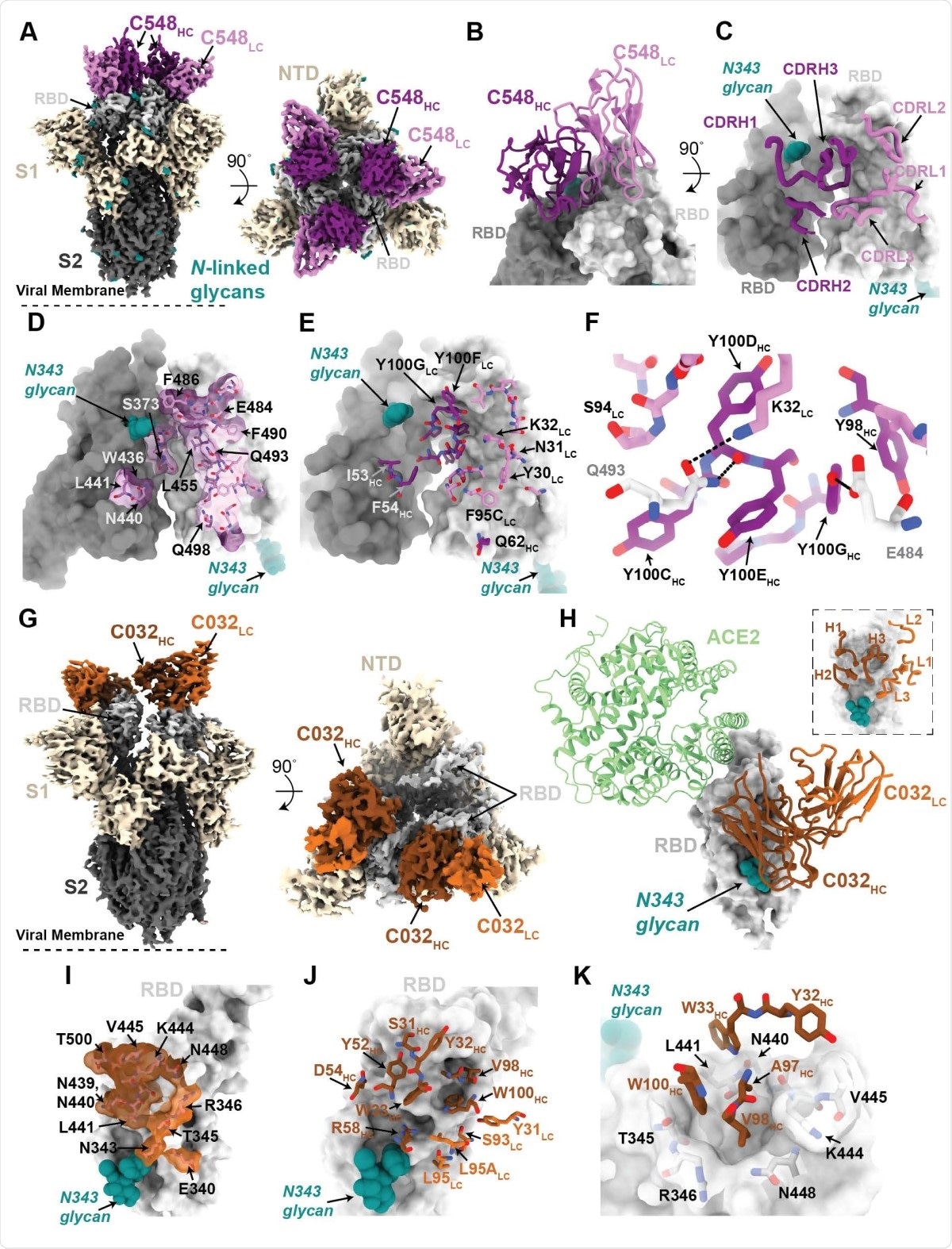
Structures of class 2 and class 3 anti-RBD 1.3m antibodies. (A) 3.4 Å cryo-EM density for class 2 C548-S complex (only the VH-VL domains of C548 are shown). (B) Close-up view of quaternary epitope involving bridging interactions between adjacent RBDs. (C) CDR loops mapped onto adjacent RBD surfaces. (D) Epitope of C548 highlighted on adjacent RBDs. (E) C548 paratope mapped onto adjacent RBDs. (F) Interactions between RBD and C548 with a subset of interacting residues highlighted as sticks. Potential hydrogen bonds shown as dotted lines. (G) 3.4 Å cryo-EM density for class 3 C032-S complex (only the VH-VL domains of C032 are shown). (H) Overlay of C032–RBD portion of the C032-S complex structure with an ACE2-RBD structure (from PDB 6VW1). (I) Epitope of C032 highlighted on the RBD surface. (J) C032 paratope mapped onto RBD surface. (K) Interactions between RBD and C032 CDRH1 and CDRH3 loops, with a subset of interacting residues highlighted as sticks. Potential hydrogen bonds shown as dotted lines.
E484K mutation lowered the neutralizing potency of mature antibodies
Given the increasing prevalence of several variants of concern, the researchers looked at it affected somatic mutations on antibodies. Three mutations found in variants of concern include K417N, N501Y, and E484K. The E484K mutation is most notable for its resistance to neutralizing antibodies from natural infection and vaccination. Therefore, researchers also exposed antibodies to the E484K substitution by itself.
The E484K substitution, generally undermines the activity of class 2 antibodies, but substitutions found in variants of concern did not impact the activity of the matured class 1 and class 3 antibodies tested herein,” writes the researchers.
The researchers suggest that other antibodies may have caused the selective pressure to mutate K417, E484, and N501 in the spike protein’s RBD.
The presence of the E484 mutation did decrease the neutralizing ability of several lineages of antibodies such as C051 and C052. The researchers note adding more mutations may not help with stopping the mutation. Although, the C549 antibody partly had neutralizing potency.
The greater neutralization potency, resilience to viral mutation, and breadth of SARS CoV-2 RBD-specific antibodies that have undergone greater degrees of somatic mutation suggests that immunization schemes that elicit higher levels of antibody mutation and diversification are desirable,” concluded the research team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Muecksch F, Development of potency, breadth and resilience to viral escape mutations in SARS-CoV-2 neutralizing antibodies. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.03.07.434227, https://www.biorxiv.org/content/10.1101/2021.03.07.434227v1
- Peer reviewed and published scientific report.
Muecksch, Frauke, Yiska Weisblum, Christopher O. Barnes, Fabian Schmidt, Dennis Schaefer-Babajew, Zijun Wang, Julio C. C. Lorenzi, et al. 2021. “Affinity Maturation of SARS-CoV-2 Neutralizing Antibodies Confers Potency, Breadth, and Resilience to Viral Escape Mutations.” Immunity 54 (8): 1853-1868.e7. https://doi.org/10.1016/j.immuni.2021.07.008. https://www.cell.com/immunity/fulltext/S1074-7613(21)00294-6.