Millions of people across the globe have been affected by the coronavirus disease 2019 (COVID-19) pandemic. Worldwide, researchers are working extensively to develop vaccines, facemasks, viral disinfectants, nasals sprays, and oral medicines to stop the spread of the respiratory pathogen, severe acute respiratory coronavirus 2 (SARS-CoV-2).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
To date, very few vaccines against SARS-CoV-2 are available. Among those that have been approved by national and international regulatory bodies are the mRNA-based vaccines (BNT162b2 and mRNA-1273) and the adenovirus-based vaccine (ChAdOx1 nCoV-19). The approved COVID-19 mRNA vaccine requires two doses, administered 3-4 weeks apart.
In many countries, volunteers were given vaccines irrespective of their previous infection by SARS-CoV-2. This method of vaccination has raised two critical questions in the scientific community. Firstly, whether or not it is necessary to vaccinate people who have already recovered from the COVID-19. Secondly, whether a second (or booster) dose of vaccine would be advantageous to immunizing this group.
A new study that has been published in medRxiv* preprint server deals with the evaluation of adaptive immune response to COVID-19 vaccination in candidates who have recovered from SARS-CoV-2 infection. The outcome of this research will have an immense impact on the current model of vaccination and would also define future clinical practices.
In this study, researchers have compared the effect of two doses of BNT162b2 mRNA COVID-19 vaccine in two groups of people. They determined the SARS-CoV-2 spike-specific T and B cells response and also studied IgG, IgM, and neutralizing antibodies specific titers in 22 individuals. Among them, 11 reported a history of COVID-19, and were regarded as “ex COVID-19”. The remaining had no history of SARS-CoV-2 infection and were categorized as “naive ones.” The demographic and clinical characteristics of all 22 subjects and their time of recovery to vaccination were recorded.
The subjects were evaluated before the administration of the vaccine. They were also tested weekly until a week after the administration of the second dose of the vaccine. This research revealed that one dose of vaccine was adequate to increase both humoral and cellular immune response in ex COVID-19 individuals. However, two doses were mandatory for naive ones.
In this study, researchers conducted experiments to determine the serum levels of the SARS-CoV-2 spike-specific antibodies of all 22 subjects. They found that the frequency of B cells capable of recognizing SARS-CoV-2 spike protein was significantly higher in ex COVID-19 subjects even before vaccination compared to the naive individuals. They also investigated the antigen-specific CD4+ T cell response to SARS-CoV-2 spike protein peptides. Additionally, they have also evaluated CD154 expression and the production of IL-2, IFN-γ, and TNF-α upon in vitro stimulation. Similar results to B cell response were obtained. The researchers found the presence of SARS-CoV-2 spike-specific CD4+ T cells in ex COVID-19 subjects before vaccination, and this was absent in naive individuals.
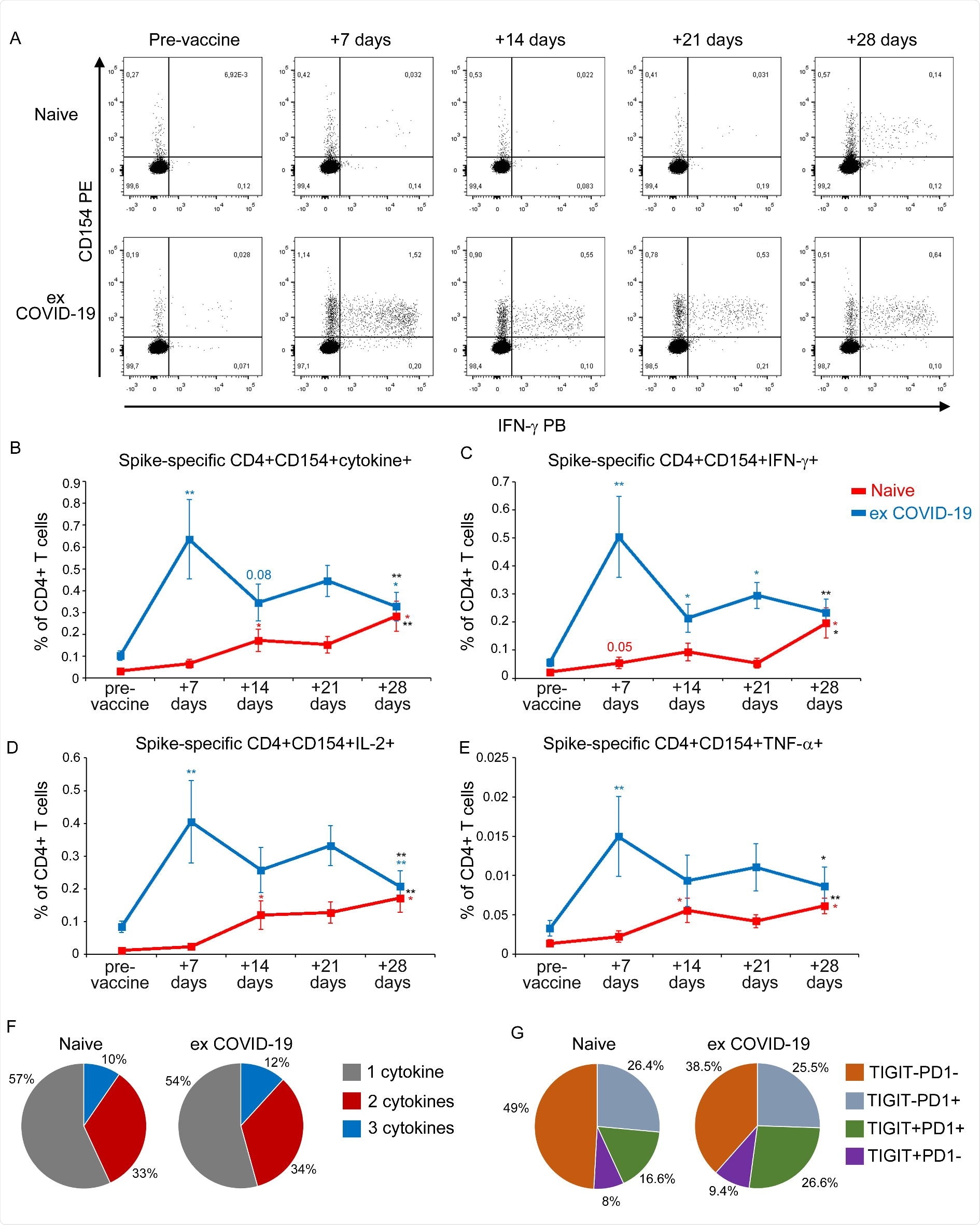
Detection of Spike-specific circulating CD4+ T cells in naïve and ex COVID19 vaccinated subjects A) Representative flow cytometric plots of Spike-specific CD4+ CD154+ IFN-γ+ T cells in one naïve (upper row) and one ex COVID-19 (lower row) subject before vaccination and after 7, 14, 21, 28 days from the first injection. Kinetic analysis of frequencies of CD154+ cells producing at least one cytokine among IL-2, IFN-γ and TNF-α(B), CD154+ IFN-γ+ (C), CD154+ IL-2+ (D), CD154+ TNF-α+ (E) Spike-specific T cells in naïve (red lines) and ex COVID-19 (blue lines) subjects, before and after vaccination. Data are represented as mean ±SE from 11 naïve and 11 ex COVID-19 subjects, subtracted of background unstimulated negative control. Blue and red asterisks refer to paired statistics within each study group compared to the previous time point in the kinetic. Black asterisks at day 28 represent paired statistic compared to pre-vaccine point. *= p<0.05; **= p<0.01; ***= p<0.001. (F) Characterization of SARS-CoV-2 Spike-specific CD4+ T cell polyfunctionality in naïve and ex COVID-19 subjects at day 28 following the first vaccine injection. Results are expressed as mean percentages from 11 naïve and 11 ex COVID-19 subjects. (G) Characterization of TIGIT and PD1 expression by SARS-CoV-2 Spike-specific CD4+ T cells in 7 naïve and 8 ex COVID-19 subjects. Results are expressed as mean percentages.
So far, this is the first research that has focused on the evaluation of the early kinetics of cellular immune response following two doses of BNT162b2 mRNA vaccine in both naive and ex COVID-19 subjects. Two key outcomes of the research are as follows:
- In naive subjects, a progressive increase in antibodies and B and T cells specific for SARS-CoV-2 spike protein takes place three weeks following the first vaccine dose. After the first dose, neutralizing antibody titers remained low, which spiked after the second jab. This result proves the importance of the second dose in the naive group.
- In ex COVID-19 subjects, researchers found a rapid reactivation of both cellular and humoral immunological memory to SARS-CoV-2 spike protein. After 7 days of the first injection, researchers observed a maximum increase in circulating spike-specific antibodies and B and T cells. However, after 14 days of administering the first vaccine dose, a decrease in the incidence of SARS-CoV-2 spike-specific CD4+ T cells was observed. As stated before, the second dose of the vaccine was found to be ineffective for ex COVID-19 subjects.
Previous research has shown the strength of the immune response to the COVID -19 virus directly correlates with disease severity in terms of both the acute phase and the memory phase. The memory immune response to SARS-CoV-2 is quite heterogeneous in the months after recovery. Therefore, more research is required to understand whether administering a single dose of the vaccine would be adequate for people with a history of asymptomatic SARS-CoV-2 infection.
This study raises the vital question of whether the second dose should be administered to ex COVID-19 subjects or redirected to naive subjects. Saving vaccine doses could substantially increase the number of doses available to naive individuals.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Alessio Mazzoni, Nicoletta Di Lauria, Laura Maggi, Lorenzo Salvati, Anna Vanni, Manuela Capone, Giulia Lamacchia,Elisabetta Mantengoli, Michele Spinicci, Lorenzo Zammarchi, Seble Tekle Kiros, Maria Grazia Colao, Paola Parronchi, Cristina Scaletti, Lucia Turco, Francesco Liotta, Gian Maria Rossolini, Lorenzo Cosmi, Alessandro Bartoloni, Francesco Annunziato, First dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in ex COVID-19 subjects. medRxiv 2021.03.05.21252590; doi: https://doi.org/10.1101/2021.03.05.21252590, https://www.medrxiv.org/content/10.1101/2021.03.05.21252590v1
- Peer reviewed and published scientific report.
Mazzoni, Alessio, Nicoletta Di Lauria, Laura Maggi, Lorenzo Salvati, Anna Vanni, Manuela Capone, Giulia Lamacchia, et al. 2021. “First-Dose MRNA Vaccination Is Sufficient to Reactivate Immunological Memory to SARS-CoV-2 in Recovered COVID-19 Subjects.” The Journal of Clinical Investigation, May. https://doi.org/10.1172/JCI149150. https://www.jci.org/articles/view/149150.