Extensive testing for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been a primary mitigation measure in controlling the ongoing pandemic. The infection causes the coronavirus disease 2019 (COVID-19), which has so far claimed over 2.7 million lives worldwide. The need for effective therapeutic solutions is great, even with the ongoing vaccination programs against SARS-CoV-2 in many parts of the world. One such plasma-based therapeutic approach is using neutralizing antibodies from the blood of convalescent patients.
To address the need for a high-quality neutralization assay against SARS-CoV-2, researchers from the National Institute of Allergy and Infectious Diseases (NIAID) modified and optimized a previously established fluorescence reduction neutralization assay (FRNA) to provide a qualitative assessment of a large number of infected cells through the use of a high-content imaging system.
In this study, the team described a semi-high throughput, highly quantitative neutralization assay for SARS-CoV-2 built around the Operetta high-content imaging system. The authors claimed that similar equipment (which may not be available in many laboratory settings) could become equally effective if appropriately validated.
They reported that by February 2021, the SARS-CoV-2 FRNA had been used to screen over 5,000 samples, including acute and convalescent plasma or serum samples and therapeutic antibody treatments, for the SARS-CoV-2 neutralizing titers.
While there will always be variability in live-virus assays due to the nature of the biological system, using a rigorous statistical approach to inform acceptance of data can mitigate the potential negative effects of poor infection efficiency, pipetting errors, edge effects, and inconsistent staining.”
COVID-19 is a heterogeneous disease, often manifesting severe respiratory disease, cardiovascular disease, and neurological disease. A rapid antibody class switch from immunoglobulin M (IgM) to IgG and IgA occurs during acute disease. The researchers note that the antibody isotype is important in controlling the disease, as is the target viral protein.
The researchers observed that disease severity is associated with more robust and prolonged antibody responses to the viral nucleoprotein (N). Thus the presence of anti-SARS-CoV-2 antibodies in the blood is presumed to be a good measure of protective immunity for a vaccine candidate. This calls for rapid methods that are reliable and sensitive to detect SARS-CoV-2 neutralizing antibodies.
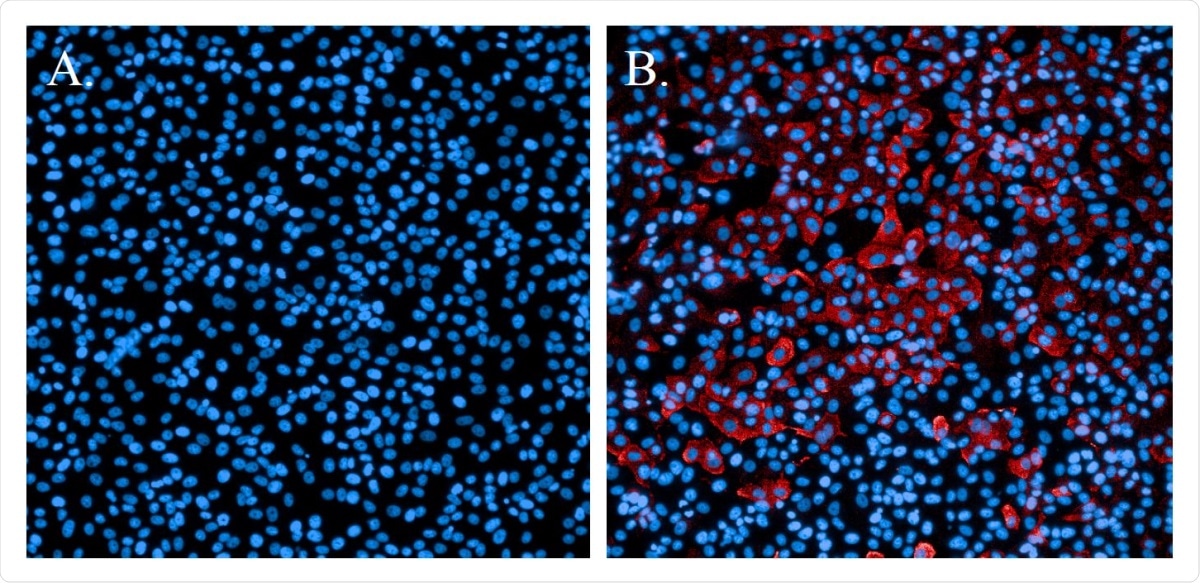
Immunofluorescence staining of SARS-CoV-2-infected cells. A. Non-infected cells stained with Hoechst nuclear stain (blue). B. Cells infected with SARS-CoV-2 and probed with a SARS-CoV N-protein-specific antibody and Alexa594 secondary antibody (red). Cells were counterstained with Hoechst nuclear stain (blue).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The FRNA is a highly specific, quantifiable and rigorous assay to evaluate neutralizing antibodies in a test sample. The assay quantifies individual cells that are infected with the initial addition of the virus and do not require multiple rounds of viral replication.
As an atypical assay, it does not rely on the subjective determination of cell cytopathic effects nor the development of multicellular plaques or immunofoci using a low number of infectious particles per well. The researchers tested for outliers in the virus control and cell control observations and evaluated the variability and specificity of the assay.
Because the assay uses 12-step dilution schemes and four-parameter logistical analysis to quantify a specific NT50, it provides a more precise understanding of neutralization capacity, particularly in the case of monoclonal antibodies or nanobodies.
In this study, the researchers described the development of a semi-high-throughput SARS-CoV-2 neutralization assay that takes advantage of the capabilities of a high-content imaging system to quantify the number of infected cells in individual wells. Importantly, the researchers demonstrated that the assay has been quickly adapted for use with multiple virus variants. Such assays immensely help and accelerate the preclinical vaccine studies and clinical trials.
Initially, during the COVID-19 crisis, the U.S. FDA (Food and Drug Administration) approved an Expanded Access Program (EAP) to treat COVID-19 patients using plasma from individuals with a neutralization titer of 1:160 or higher. This program treated over 94,000 patients across the U.S. Last August, the FDA issued an Emergency Use Authorization (EUA) to allow therapeutic plasma treatment of COVID-19 patients outside the context of clinical trials.
The researchers claim that this assay is devoid of subjective interpretation and therefore more precise than most other wild-type virus neutralization assays.
“While there will always be variability in live-virus assays due to the nature of the biological system, using a rigorous statistical approach to inform acceptance of data can mitigate the potential negative effects of poor infection efficiency, pipetting errors, edge effects, and inconsistent staining.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Richard S. Bennett, Elena N. Postnikova, Janie Liang, Robin Gross, Steven Mazur, Saurabh Dixit, Vladimir V. Lukin, Greg Kocher, Shuiqing Yu, Shalamar Georgia-Clark, Dawn Gerhardt, Yingyun Cai, Lindsay Marron, Michael R. Holbrook (2021) Scalable, Micro-Neutralization Assay for Qualitative Assessment of SARS-CoV-2 (COVID-19) Virus-Neutralizing Antibodies in Human Clinical Samples. bioRxiv 2021.03.05.434152; doi: https://doi.org/10.1101/2021.03.05.434152, https://www.biorxiv.org/content/10.1101/2021.03.05.434152v1
- Peer reviewed and published scientific report.
Bennett, Richard S., Elena N. Postnikova, Janie Liang, Robin Gross, Steven Mazur, Saurabh Dixit, Gregory Kocher, et al. 2021. “Scalable, Micro-Neutralization Assay for Assessment of SARS-CoV-2 (COVID-19) Virus-Neutralizing Antibodies in Human Clinical Samples.” Viruses 13 (5): 893. https://doi.org/10.3390/v13050893. https://www.mdpi.com/1999-4915/13/5/893.